Introduction
Memory encompasses the storage of information,
the mental representation of experiences and the
mental processes involved in learning, storing
and retrieving information [1]. Human memory
can be categorized into three essential types:
Sensory memory, short-term memory and long-
term memory [2]. Information processing begins
in sensory memory, where it is held for a brief
moment. It then transitions to short-term memory,
which serves as the subject’s working memory
and may eventually be consolidated into long-
term memory [2].
Memory loss, a central and highly feared
consequence of aging, is also the signal symptom
of dementia [3]. Memory issues, such as poor recall, low retention, difficulty concentrating and
weak analytical skills and are prevalent in today’s
world [4]. In some cases, these issues may escalate
into more serious conditions, such as Alzheimer’s
Disease (AD) or schizophrenia [4,5]. Memory loss
is often one of the initial symptoms of AD reported
by patients with and their caretakers [6]. As of
2019, the global prevalence of AD was estimated
at 57.4 million cases and this number could rise
to 152.8 million by 2050 [7]. The increasing
prevalence of AD, coupled with its strong impact
on individuals and families, highlights the critical
need for effective treatments and preventive
strategies for AD and memory loss.
Nootropics, commonly referred to as
smart drugs, encompass a diverse class of
pharmacological agents that enhance cognitive functions, including thinking, learning and
memory, particularly in conditions where these
capabilities are impaired [8]. Nootropics work
by enhancing the brain’s glucose and oxygen
supply, exhibiting antihypoxic properties and
protecting brain tissue from neurotoxicity [8-10].
Classical Nootropic Compounds include Deanol
Dimethylaminoethanol (DMAE), Meclofenoxate,
Nicergoline, Piracetam and Pyritinol [8].
Additionally, some substances exhibit
simultaneous nootropic, hemorheological and
vasodilatory effects. Examples of these include
vinpocetine, naftidrofuryl and dihydroergotoxine
[8]. While there are benefit of nootropic drugs
and nootropics are generally well tolerated and
typically mild, there are still concerns over side
effects, addictiveness and potential abuse of
drugs [11,12]. Meanwhile, options to explore
natural substances based on the traditional use
have been regained interest as an alternative to
those modern medicines. For example, traditional
systems such as Ayurveda, have identified several
herbs and plants with memory benefits [13,14].
Several species of plants have been selected for
testing as nootropic agents because of their use in
traditional medicine. Notable examples include Gingko biloba, Panax ginseng, Paullinia cupana
and Rhodiola rosea [8].
Southeast Asia, with its rich biodiversity and
traditional medicinal practices, offers a plethora of
natural substances that could potentially enhance
memory. However, there is a lack of comprehensive
literature reviews summarizing the use of natural
substances for memory enhancement in Southeast
Asian regions. This review explores natural
substances from this region, summarizing their
taxonomic classification, the parts used, traditional
applications and the scientific evidence supporting
their potential for memory enhancement and their
role as alternatives to nootropics. The bioactive
compounds of highlighted substances are also
discussed.
Methodology
Through collaborations with Southeast Asian
research partners, Delightex is exploring natural
substances that may enhance mental well-being,
in this review, we have refereed to works done
by Delightex’s research partners from Philippines
(the University of Santo Tomas, the University
of Antique) and Delightex’s Vietnam research team. The criteria of natural substance search
using key words such as ‘natural substances,’ ‘Southeast Asian countries’ and ‘medicinal plants’ using databases and other online sources. Based
on the broad search on mental wellness, we
further narrowed specific natural substances with
potential memory-enhancing effects, utilizing
terms such as ‘memory enhancement,’ “memory
boosting’, ‘memory impairment,’ ‘memory loss’ and ‘nootropics.’ This review discusses selected
plants traditionally used or supported by scientific
evidence for memory enhancement in Southeast
Asian countries.
Results and Discussion
Following comprehensive reviews of mental well- being enhancing natural substances conducted across Southeast Asia by Delightex’s research partners, including the University of Santo Tomas, the University of Antique and Delightex’s research team in Vietnam, we further focused on identifying natural substances with memory- enhancing properties. This investigation led to the identification of 57 natural substances (Table 1). The table provides detailed information for each substance, including its botanical family, scientific name, utilized part, traditional applications (if available) and supporting scientific evidence for its memory improvement effects.
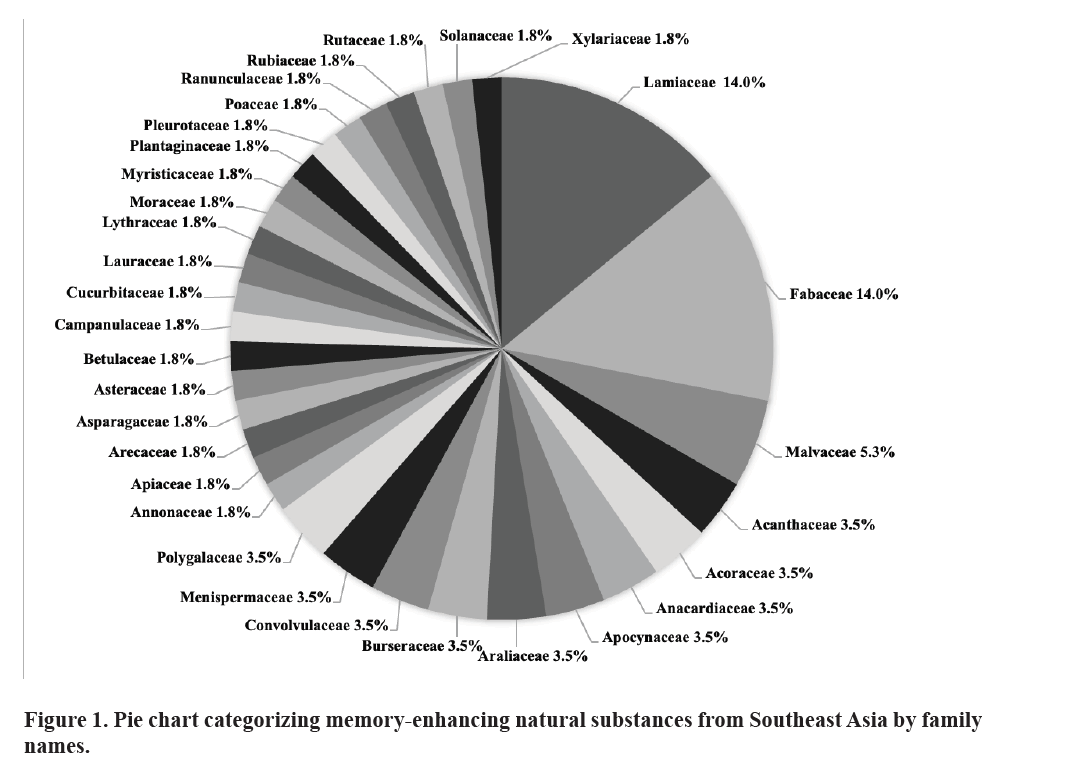
In this review, the substances have been categorized and analyzed by botanical family to offer a more comprehensive understanding of their potential roles in promoting mental well- being. Figure 1 depicts the distribution of memory enhancing natural substances in Southeast Asia by families. 57 memory enhancing natural substances are from 31 families. Among them, 15.8% natural substances are from the Lamiaceae family, followed by the Fabaceae family (14.0%) and the Malvaceae family (5.3%).
Plant families
The Lamiaceae family, also known as the mint or deadnettle family, comprises dicotyledonous flowering plants distributed globally. The enlarged Lamiaceae contains about 236 genera and about 6900 to 7200 species [15]. The Lamiaceae family is known for its high essential oil content and is also rich in polyphenolic compounds and terpenoids [16]. This review has listed 8 substances within this family (Table 1).
Hyptis suaveolens, commonly known as pokok kemangi in Malaysia, amotan, suobkabayo, or loko- loko in the Philippines and kara or maeng lak kha in Thailand [17]. It is found in southeast countries such as Cambodia, Thailand, Indonesia, Malaysia, Vietnam and Philippines [18]. H. suaveolens is a rich source of medicinally significant phytochemicals, including essential oils, tannins, saponins, phenols, flavonoids, terpenoids, alkaloids and sterols. Main components identified in H. suaveolens essential oil include 1,8-Cineole (Eucalyptol), Sabinene, β-Caryophyllene and β-elemene [19]. These compounds belong to the terpene classes of monoterpene hydrocarbons and sesquiterpene hydrocarbons [19]. Traditionally, plant extracts of H. suaveolens were used as a memory aid [20]. In India, it was referred to as Chan or Wilaiti Tulsi, and the morning corn soup known as bate was believed to aid memory [21].
Salvia rosmarinus, commonly known as rosemary, is an evergreen, bushy shrub that thrives along the Mediterranean coast and in sub-Himalayan regions [22]. It is called dumero, romero and rosmiro in Philippines [23]. In Philippines, a leaf infusion of rosemary is utilized as eyewash for mild catarrhal conjunctivitis, as vapor baths for the treatment of rheumatism, paralysis and early- stage catarrhs and for bathing postpartum women [23]. The main bioactive compounds in rosemary are triterpenes, phenolic diterpenes and phenolic acids including rosmarinic acid, carnosic acid, rosmanol, carnosol, ursolic acid and betulinic acid [24,25]. In traditional medicine, rosemary is believed to fortify the brain and refresh the memory [23]. The rosemary extracts have clinical effects on mood, learning, memory, pain, anxiety and sleep. Rosemary (500 mg administered twice daily for one month) has been shown to enhance both prospective and retrospective memory in a study involving 68 university students [26]. Additionally, Rosemary powder (750 mg), a dose comparable to typical culinary use, has demonstrated positive effects on memory speed and the efficiency of retrieving information from both episodic and working memory in 28 older adults (mean age: 75 years) [27]. Their finding suggests that rosemary may be a valuable memory enhancer.
Vitex negundo, commonly known as Chinese chaste tree, is native to tropical Eastern and Southern Africa and Asia [28]. In Southeast Asia, it is indigenous to countries such as Bhutan, Cambodia, Indonesia, Malaysia, Myanmar, Philippines, Thailand and Vietnam [28]. Kanwal et al. reported that treatment with V. negundo aqueous extracts (300 mg/kg) for 5 days reduced scopolamine-induced amnesia in rats by enhancing memory and learning through antioxidant effects and decreasing AChE activity [29]. The administration of hydroalcoholic extract of V. negundo leaves at doses of 250 mg/kg and 500 mg/kg over 8 days significantly enhanced learning and memory in mice [30]. This improvement may be attributed to AchE inhibition, antioxidant activity and/or enhanced cholinergic transmission and the nootropic activity could be associated with the presence of compounds such as flavonoids, triterpenoids, phenolic acids and lignans in the extract [30].
Family Fabaceae: The Fabaceae family, commonly known as the legume, bean, or pea family, is the third largest family in the plant kingdom, comprising approximately 19,500 species, which constitute about 7% of all flowering plants [31]. This review has listed and summarized 7 substances within this family (Table 1).
Arachis hypogaea, peanut is one of the most consumed oil seeds worldwide. Peanuts have spread to other parts of the world from South America [32]. Peanuts are extensively utilized in the culinary traditions of Southeast Asia, particularly in countries such as Malaysia, Vietnam and Indonesia, where they are commonly processed into spicy sauces. In the Philippines, peanut is a key ingredient in the traditional dish kare-kare and fried shelled peanuts are widely consumed as an affordable snack throughout the Philippines. Regular peanut and peanut butter consumption may improve memory function and stress response in healthy young adults. These effects appear to be linked to the intake of peanut polyphenols [33]. In this 6-month randomized controlled trial, participants consuming 25 g/day of skin-roasted peanuts or consuming 32 g/day of peanut butter have shown improved immediate memory after the intervention [33].
Clitoria ternatea, known as butterfly pea and blue pea in various countries is originated from tropical Asia countries and it is widely available in the Asian regions [34,35]. As a traditional Ayurveda medicine as a brain tonic, memory and nootropic herb, butterfly pea flower plays an important role in nervine medicine as well as improving brain system and boosting memory [35]. Chronic oral administration of butterfly pea root extract for 28 days at doses of 200 mg/kg and 300 mg/ kg effectively restored memory impairments in rats induced by Chronic Cerebral Hypoperfusion (CCH) [36].
Pueraria candollei var. mirifica is endemic to Thailand and is widely distributed in the country’s deciduous forests, particularly at altitudes of 300 to 800 meters. It is called kwao khrua khaw (various spellings) in the Thai language [37]. It is most commonly and abundantly found in the northern, western and northeastern regions of Thailand [38,39]. The main bioactive phytochemicals of P. mirifica are phytoestrogens including isoflavonoids, coumestans and chromenes [40]. Traditionally, pounding the roots of P. mirifica and mixing them with cow’s milk is believed to enhance memory [41]. P. mirifica extracts have demonstrated beneficial effects on cognitive deficits associated with menopause or estrogen depletion, primarily through its phytoestrogenic and antioxidant properties [42]. In the same study, it was found that PM extracts at a daily dose of 25 mg/kg exerted anti-dementia effects that were almost equivalent to the effects elicited by 17β-estradiol at 1 μg/kg [42].
Family Malvaceae: The Malvaceae, commonly known as the mallow family, is a group of dicotyledonous flowering plants comprising approximately 244 genera and around 4,225 species. These species are distributed across regions ranging from tropical to temperate climates [43]. This review has explored 3 substances within this family (Table 1).
Abelmoschus manihot is in South-East Asia it is cultivated particularly in the eastern parts of Indonesia and in Papua New Guinea [44]. In North Sulawesi, Indonesia, this plant is known as “gedi” and its leaves are a key ingredient in preparing porridge, a distinctive gourmet dish in North Sulawesi cuisine [45]. More than 128 phytochemical compounds have been extracted and identified from the flowers, seeds, stems and leaves of A. manihot [46]. These compounds primarily include flavonoids, amino acids, nucleosides, polysaccharides, organic acids, steroids and volatile oils [46]. The hydroalcoholic extract of A. manihot flowers has been shown to alleviate learning and memory impairments caused by sleep deprivation in mice [47]. This effect may be attributed to its antioxidant capacity and enhanced BDNF/TrkB/GluR1 levels in the hippocampal memory [47].
Thespesia populnea commonly known as ‘Indian tulip tree. The species is native to Australia, China and India but can also be found on rocky coasts such as in Malaysia and other countries in SEA including Cambodia, Indonesia, Philippines and Thailand [48]. T. populnea contains glycosides such as quercetin, gossypol, β-sitosterol and sesquiterpene, lupenone and lupeol [49,50]. Thespesenone and dehydrooxoperezinone-6- methyl ether have been isolated from the red hardwood, while alanine, arginine, methionine and tryptophan have been identified in the seeds [51,52]. The bark of T. populnea shows potential as a memory-enhancing agent. Oral administration of ethanol bark extract of T. populnea at doses of 200 mg/kg and 400 mg/kg for 7 days significantly improved memory in both young and aged mice [53].
Other families: Areca catechu, belongs to the Arecaceae family, a species of palm native to the Philippines, is primarily grown for its areca nuts [54,55]. It is known by various common names in English, including betelnut palm, arecanut, betel palm, betel-nut, supari palm, pinang palm [56]. It is extensively cultivated in several Southeast Asian countries, including Malaysia, Indonesia, Cambodia, Laos, Myanmar, Thailand and Vietnam [57]. A. catechu contains alkaloids, polyphenols, polysaccharides, triterpenes, steroids, fatty acids and other components [58]. These compounds demonstrate a variety of bioactive functions, such as antibacterial, deworming, antiviral, antioxidant, anti-inflammatory and anti-tumour effects [58]. Extracts of arecanut have been reported to improve memory. Oral administration of methanol extracts of arecanut at a dose of 500 mg/kg for 21 days significantly improved memory and learning in rats [59]. Arecoline, the major alkaloid of arecanut, has been reported to enhance memory in both laboratory animals and humans, as summarized by Bhat et al [60]. For example, in humans, a 4 mg injection of arecoline significantly enhanced serial learning and reversed impaired learning behaviour induced by scopolamine, a cholinergic antagonist [61]. Additionally, a 4 mg dose of arecoline significantly improved picture recognition in Alzheimer’s patients [62].
Bacopa monnieri also known as waterhyssop, thyme-leafed gratiola, bacopa and brahmi, is a perennial, creeping herb from the Plantaginaceae family. Bacopa is a medicinal herb native to South and Southeast Asia [63]. Ayurvedic medicine classifies Bacopa as a ‘medhya rasayana’ which refers to a category of herbs thought to enhance mental health, boost memory and intellect and support rejuvenation and longevity [64,65]. Preliminary clinical studies have suggested that Bacopa has the potential in ameliorating cognitive disorders, as well as prophylactic reduction of oxidative damage, neurotransmitter modulation and cognitive enhancement in healthy individuals [66]. Additionally, across six human randomized controlled trials were all conducted over 12 weeks have reported that 300 mg to 450 mg bacopa extract per day have improved performance in the domain of memory free recall [67]. B. monnieri is rich in a variety of distinct bioactive phytoconstituents, each offering a range of benefits. Among them, Bacopaside XI, bacopaside I and bacopasaponsin C, administered at a concentration of 50 mg/kg, demonstrated nootropic effects and reduced memory impairment in mice [68,69].
Table 1. List of natural substances in Southeast Asia countries and their usage for memory enhancement.
| Family |
Scientific name |
Part |
Scientific evidence and traditional knowledge (if available) on memory enhancement |
References |
| Acanthaceae |
Peristrophe bicalyculata |
Leaf |
The methanolic leaf extract improves memory performance in a rat model of type 2 diabetes mellitus |
[70] |
| Rhinacanthus nasutus |
Leaf, root |
The ethanol extracts from the leaves and roots have demonstrated a dose-dependent ability to reduce neuron cell death induced by both glutamate and amyloid-β exposure |
[71] |
| Acoraceae |
Acorus calamus |
Root, Rhizome |
In Ayurvedic medicine, rhizomes are used for treatment of epilepsy, schizophrenia and memory disorders The memory impairment in groups that received aqueous fraction at 600 mg/kg dose, was less than control Alpha-asarone, as a major component of the A. calamus ameliorates memory deficit in mice |
[72-74] |
| Acorus gramineus |
Rhizome |
Across 34 studies involving 1,431 animals, extracts or active components was found to significantly enhance learning and memory function |
[75] |
| Anacardiaceae |
Rhus verniciflua |
Bark |
Active component fesetin reduced memory deficits induced by scopolamine through activation of the CREB–BDNF pathway |
[76] |
| Spondias mombin |
Leaf |
Oral administration of aqueous extracts at 400mg/kg for 28 days improved learning and memory capabilities in rats |
[77] |
| Annonaceae |
Annona squamosa |
Leaf |
The leaf extract and its isolated constituent-anonaine demonstrated memory boosting and memory regaining effects in rats |
[78] |
| Apiaceae |
Centella asiatica |
N/A |
An Ayurvedic herb used to enhance memory and nerve function Water extracts (200 mg/kg/day, 2 weeks) attenuated amyloid-β-associated behavioral abnormalities in the Tg2576 mouse, a murine model of AD |
[79] |
| Apocynaceae |
Catharanthus roseus |
Leaf, stem and root |
Aqueous extract of leaf, stem and root have been shown to effectively inhibit AchE in-vitro and active compound serpentine displayed a strong activity against Acetylcholinesterase (AchE) in-vitro, suggesting a potential as a therapeutic agent for AD |
[80] |
| Tabernaemontana divaricata |
Root |
The ethanolic root extract acts as a reversible Acetylcholinesterase Inhibitor (AChE-I) and may offer potential as a novel therapeutic agent for AD |
[81] |
| Araliaceae |
Eleutherococcus senticosus |
Leaf |
Oral administration of the water extract for 17 days significantly enhanced object recognition memory pure compounds ciwujianoside C3, eleutheroside M, ciwujianoside B were administered orally for 17 days to normal mice and significantly enhanced object recognition memory |
[82] |
| Panax ginseng |
Root |
Oral administration of fermented cultured wild ginseng root extract (HLJG0701-β) (250 mg/kg, 8 weeks) resulted in memory improvement in mice |
[83] |
| Arecaceae |
Areca catechu |
Fruit, seed, nut |
Refer to the text above |
| Asparagaceae |
Asparagus racemosus Willd |
Root |
Administration of extract (200 mg/kg, 7 days) has shown a promising memory-enhancing effect in both young and aged mice |
[84] |
| Asteraceae |
Achillea biebersteinii |
Aerial Part (stem, leaf and flower) |
Inhalation of essential oil (1%, 3%) for 21 days significantly increased spontaneous alternation percentage, and decreased the working memory errors and reference memory errors in rats |
[85] |
| Betulaceae |
Betula platyphylla |
Bark |
Oral treatment using bark (BPB-316) significantly attenuated amyloid-β-induced memory impairment in mice |
[86] |
| Burseraceae |
Commiphora caudata |
Leaf |
Ethanolic leaves extract (200 mg/kg, 400 mg/kg, 27 days) enhanced learning and memory activity: Transfer Latency (TL), Time Taken To Reach Reward Chamber (TRC) and Swim Latency (SL) in comparison to scopolamine treated rats |
[87] |
| Garuga pinnata |
Bark |
Contains bioactive phytoconstituents, including alkaloids, tannins, phenols, and flavonoids, which possess free radical scavenging properties and memory-enhancing capabilities |
[88] |
| Campanulaceae |
Platycodon grandiflorus |
Root |
Administration of root to mice resulted in increased spontaneous alternation in the Y-maze test and promoted synaptogenesis in the hippocampus |
[89] |
| Convolvulaceae |
Convolvulus pluricaulis |
Whole plant |
Administration of extract (200 mg/kg, 7 days) has shown a promising memory-enhancing effect in both young and aged mice |
[84] |
| Ipomoea batatas |
Tuber |
Anthocyanins extracted from purple sweet potato demonstrate memory-enhancing effects, potentially linked to their antioxidant properties |
[90] |
| Cucurbitaceae |
Cucurbita maxima |
Seed |
Ethanolic seed extract (50 mg/kg, 100 mg/kg and 200 mg/kg) exhibited anxiolytic and antidepressant effects with memory improvement in mice |
[91] |
| Fabaceae |
Mimosa pudica |
Leaf |
Behavioral effects on learning and memory were augmented by leaf ethyl acetate extracts (200 mg/kg and 400 mg/kg) |
[92] |
| Albizia adianthifolia |
Leaf |
Aqueous extract (150 mg/kg, 300 mg/kg) plays an important role in spatial memory formation, especially on working and reference memories |
[93] |
| Arachis hypogaea |
Seed, leaf |
Refer to the text above |
| Cassia obtusifolia/ Senna obtusifolia |
Seed |
Ethanolic seed extract (50 mg/kg) attenuates memory impairment induced by scopolamine or 2VO and that these effects are mediated by enhancing the cholinergic nervous system via acetylcholinesterase inhibition |
[94] |
| Clitoria ternatea |
Root, whole plant |
Refer to the text above |
| Glycyrrhiza glabra |
Root |
Aqueous extracts (150 mg/kg ) significantly improved learning and memory of mice |
[95] |
| Piliostigma thonningii |
Stem, Bark |
Aqueous and methanolic stem bark extract showed remarkable cognitive-enhancing activities which were reflected in significantly shorter transfer latencies, navigation distances, longer time spent in platform quadrant, and lower MDA levels compared to the negative control mice |
[96] |
| Pueraria candollei var. mirifica |
Roots, tuber |
Refer to the text above |
| Lamiaceae |
Hyptis suaveolens |
Whole plant (Except root) |
Refer to the text above |
| Melissa officinalis |
Leaf |
Administration of ethanolic extract (200 mg/kg) could significantly enhance learning and memory in memory-impaired rats |
[97] |
| Prunella vulgaris var. lilacina |
Flower |
Ethanolic flower extract (25 mg/kg) significantly shortened escape latencies in training-trials. Furthermore, swimming times within the target zone during the probe-trial were significantly increased as compared with scopolamine-treated mice |
[98] |
| Salvia rosmarinus |
Leaf |
Refer to the text above |
| Salvia miltiorrhiza |
Root |
Subchronic administration of root extract (200 mg/kg) led to an improvement of long-term memory of rats |
[99] |
| Salvia officinalis |
Leaf |
S. officinalis aroma group performed significantly better than the control group on the quality of memory and secondary memory primary outcome factors from the test battery |
[100] |
| |
Scutellaria baicalensis |
Root |
Extracts (30 mg/kg) improved spatial memory functions and rescued neuronal cells immune-reactive to ChAT and the NMDA receptor subunit, NR2A, in the hippocampus of memory deficient rat model |
[101] |
| Vitex negundo |
Leaf |
Refer to the text above |
| Lauraceae |
Cinnamomum zeylanicum |
Bark |
Aqueous bark extract-treated animals exhibited an improved discrimination between a familiar object and a novel object, indicating the reversal of extract induced memory impairment. Extract also restored alteration in AChE activity and oxidative stress parameters in both brain parts |
[102] |
| Lythraceae |
Lawsonia inermis |
Leaf |
Extract at doses of 200 mg/kg and 400 mg/kg significant increased the inflexion ratio in elevated plus maze and increase in percentage alternation in Y-maze model compared to negative control animals |
[103] |
| Malvaceae |
Abelmoschus manihot |
Flower |
Refer to the text above |
| Abelmoschus moschatus |
Seed |
Oral administration of ethanolic seed extract (100 mg/kg, 200 mg/kg) for seven days demonstrated a dose-dependent improvement in memory in young mice and effectively reversed diazepam-induced memory deficits |
[104] |
| Thespesia populnea. |
Bark |
Refer to the text above |
| Menispermaceae |
Cissampelos pariera |
Root |
The dose of 400mg/kg of CPE significantly improved learning and memory of mice |
[105] |
| Tiliacora triandra |
Leaf |
Leaf extract at doses of 300 mg/kg and 600 mg/kg significantly enhances spatial learning and learning flexibility. Only the 300 mg/kg dose showed a significant improvement in spatial memory |
[106] |
| Moraceae |
Ficus racemosa |
Bark |
Administration of the extract at two levels, 250 mg/kg and 500 mg/kg extract resulted in significant reduction in transfer latency on elevated plus-maze, which was used as an exteroceptive behavioural model to evaluate memory in rats |
[107] |
| Myristicaceae |
Myristica fragrans |
Seed |
Extract at the lowest dose of 5 mg/kg P.O. administered for 3 successive days significantly improved learning and memory of young and aged mice |
[108] |
| Plantaginaceae |
Bacopa monnieri |
Whole plant/leaf |
Refer to the text above |
| Poaceae |
Cymbopogon citratusx |
Leaf |
After the inhalation, the lemongrass essential oil enhanced their cognitive performance for the domains of the continuity of attention and the quality of memory, whereas the mood in terms of alertness and calmness was also increased |
[109] |
| Pleurotaceae |
Pleurotus eryngii |
Fruiting Body |
The ethanol extract of P. ryngii exhibits estrogen-like effects, providing potential benefits for alleviating depression and improving memory impairment in rats Feeding with P. eryngii for six weeks has promoted memory and learning capacity in an AD mouse model |
[110, 111] |
| Polygalaceae |
Polygala japonica |
Root |
A traditional medicine for treatment of variety of ailments, including anti-inflammatory, antibacterial, sedative and nootropic agent Compound saponins can improve the learning and memory in mice |
[112] |
| Polygala tenuifolia |
Root |
The impaired spatial memory of the aged mice was partly reversed by extract (100 mg/kg and 200 mg/kg) as compared with the aged control mice. In stepdown tests, the nonspatial memory of the aged mice was improved by extract (100 mg/kg and 200 mg/kg) |
[113] |
| Ranunculaceae |
Nigella sativa |
Seed |
Hydro-alcoholic extract of NS improved the LPS-induced learning and memory impairments in rats |
[114] |
| Rutaceae |
Lunasia amara |
Bark |
A traditional Ayurveda medicine as a brain tonic, memory and intelligence enhancer |
N/A |
| Solanaceae |
Withania somnifera |
Root |
Known as Ashwagandha, it has been traditionally used in Ayurvedic medicine as a substance to strengthen the nervous system Root extract alleviated hypobaric hypoxia -induced memory impairment and neurodegeneration in the hippocampus by modulating corticosterone levels through nitric oxide pathways |
[115,116] |
| Xylariaceae |
Xylaria nigripes |
Mycelium |
Water extracts (300 mg/kg/day, 6 weeks) improved Rapid Eye Movement Sleep Deprivation (REMSD)-induced memory impairment in rats |
[117] |
| Rubiaceae |
Morinda citrifolia |
Fruit |
Extract prevented memory impairment induced by amyloid-β peptide in mice |
[118,119] |
Conclusion
Southeast Asia, with its rich tradition of medicinal
practices and reliance on natural remedies, offers
a diverse array of natural substances with potential
memory-enhancing properties. This review has
summarized 57 natural substances that originate
from, are cultivated in, or are traditionally used
in the region, many of which could serve as
alternatives to nootropics or have already been
incorporated into nootropic formulations. These
substances span 31 different families such as the
Lamiaceae, Fabaceae and Malvaceae families. The
review discusses about the traditional knowledge
and scientific evidence related to their memory-
enhancing effects While most evidence is derived
from animal studies, human clinical studies of
effect of rosemary (S. rosmarinus) peanut (A.
hypogaea), arecanut (Areca catechu) and Bacopa
(Bacopa monnieri) are also discussed. This review
also discusses the bioactive compounds of the
highlighted substances. However, the specific
compounds responsible for memory enhancement
and their underlying pharmacological mechanisms
in many natural substances remain unclear. Further
research is needed to elucidate these mechanisms,
identify the compounds responsible for memory
enhancement, evaluate their safety profiles and
explore the potential of these natural substances
as alternatives to existing nootropics.
Acknowledgment
The authors would like to express their deepest
gratitude to Delightex’s research partners from the
University of Santo Tomas, University of Antique
and Delightex’s Vietnam research team for their
invaluable assistance in providing literature search
on mental well-being substances in Southeast
Asian countries.
References
- Radvansky GA. Human memory. Routledge. 2021.
[Crossref] [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control process. Psychol Learn Motiv-Adv Res Theory. 1968;2:189-195.
[Crossref] [Google Scholar]
- Andrew B, Paul S. Memory loss: A practical guide for clinicians. 2011.
[Google Scholar]
- Halder S, Anand U, Nandy S, Oleksak P, Qusti S, et al. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitive and brain booster’s perspectives. Saudi Pharm J. 2021;29(8):879-907.
[Crossref] [Google Scholar] [PubMed]
- Gur RC, Gur RE. Memory in health and in schizophrenia. Dialogues Clin Neurosci. 2013;15(4):399-410.
[Crossref] [Google Scholar] [PubMed]
- Jahn H. Memory loss in Alzheimer's disease. Dialogues in clinical neuroscience. 2013;15(4):445-454.
[Crossref] [Google Scholar] [PubMed]
- Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e125.
[Crossref] [Google Scholar] [PubMed]
- Malik M, Tlustos P. Nootropics as cognitive enhancers: Types, dosage and side effects of smart drugs. Nutrients. 2022;14(16):3367.
[Crossref] [Google Scholar] [PubMed]
- Joshi Pranav C. A review on natural memory enhancers (Nootropics). Unique Journal of Engineering and Advanced Sciences. 2013;1(01):8-18.
[Google Scholar]
- Malik R, Sangwan A, Saihgal R, Paul Jindal D, Piplani P. Towards better brain management: nootropics. Curr Med Chem. 2007;14(2):123-131.
[Crossref] [Google Scholar] [PubMed]
- Schifano F, Catalani V, Sharif S, Napoletano F, Corkery JM, et al. Benefits and harms of ‘smart drugs’(nootropics) in healthy individuals. Drugs. 2022;82(6):633-647. 1.
[Crossref] [Google Scholar] [PubMed]
- Garcia HS, Reula LM, Fernandez AP, Sanchez VP, Lopez NO, et al. Nootropics: Emergents drugs associated with new clinical challenges. Eur Psychiatr. 2017;41(S1):s877-s878. 1.
[Crossref] [Google Scholar]
- Farooqui AA, Farooqui T, Madan A, Ong JH, Ong WY. Ayurvedic medicine for the treatment of dementia: Mechanistic aspects. Evid Based Complement Alternat Med. 2018;2018(1):2481076.
[Crossref] [Google Scholar] [PubMed]
- Mehla J, Gupta P, Pahuja M, Diwan D, Diksha D. Indian medicinal herbs and formulations for Alzheimer’s disease, from traditional knowledge to scientific assessment. Brain Sci. 2020;10(12):964.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Deng M. Identification and Control of Common Weeds: Volume 2. Dordrecht: Springer Netherlands; 2017.
[Crossref] [Google Scholar]
- Abdelhalim A, Hanrahan J. Biologically active compounds from Lamiaceae family: Central nervous system effects. Studies in natural products chemistry. 2021;68:255-315.
[Crossref] [Google Scholar]
- Seidemann J. World spice plants. Berlin, Heidelberg: Springer Berlin Heidelberg. 2005.
[Crossref] [Google Scholar]
- Li R, Tang G, Liu X, Li J, Wang D, et al. An ethnopharmacological review of Hyptis suaveolens (L.) Poit. Trop J Pharm Res. 2020;19(7):1541-1550. 1.
[Crossref] [Google Scholar]
- Aguele FO, Oke EO, Abam FI, Nnabodo D, Agbana AS. Biological and antioxidant activities, extraction methodology and prospects of essential oil from Hyptis suaveolens (L.): A review. Clean Eng Technol. 2023;17:100685. 1.
[Crossref] [Google Scholar]
- Sharma PP, Roy RK, Anurag DG, Vipin KS. Hyptis suaveolens (L.) poit: A phyto-pharmacological review. Int J Chem Pharm Sci. 2013;4(1):1-11.
[Google Scholar]
- Perusomula R, Thamannagari S, Paturi G, Alluri R. Evaluation of anti-arthritic activity of Hyptis suaveolens seeds in rats. GSC Biol Pharm Sci. 2024;27(3):077-98. 1.
[Crossref] [Google Scholar]
- Rahbardar MG, Hosseinzadeh H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran J Basic Med Sci. 2020;23(9):1100-1112.
[Crossref] [Google Scholar] [PubMed]
- De Guzman CC, Siemonsma JS. Plant resources of South-East Asia. Backhuys Publ. 1999.
[Google Scholar]
- Borrás-Linares I, StojanoviÄ? Z, Quirantes-Piné R, Arráez-Román D, Švarc-GajiÄ? J, et al. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int J Mol Sci. 2014;15(11):20585-20606.
[Crossref] [Google Scholar] [PubMed]
- Okamura N, Fujimoto Y, Kuwabara S, Yagi A. High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis and Salvia officinalis. J Chromatogr A. 1994;679(2):381-386.
[Crossref] [Google Scholar]
- Nematolahi P, Mehrabani M, Karami-Mohajeri S, Dabaghzadeh F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement Ther Clin Pract. 2018;30:24-28.
[Crossref] [Google Scholar]
- Pengelly A, Snow J, Mills SY, Scholey A, Wesnes K, et al. Short-term study on the effects of rosemary on cognitive function in an elderly population. J Med Food. 2012;15(1):10-17.
[Crossref] [Google Scholar] [PubMed]
- Vitex negundo L. Germplasm resources information network. Agricultural Research Service, United States Department of Agriculture. 2011.
- Kanwal A, Mehla J, Kuncha M, Naidu VG, Gupta YK, et al. Anti-amnesic activity of Vitex negundo in scopolamine induced amnesia in rats. Pharmacology & Pharmacy. 2010;1(01):1.
[Google Scholar]
- Otari KV, Bichewar OG, Shete RV, Upasani CD. Effect of hydroalcoholic extract of Vitex negundo Linn. Leaves on learning and memory in normal and cognitive deficit mice. Asian Pac J Trop Biomed. 2012 ;2(1):S104-S1011.
[Crossref] [Google Scholar]
- Raj SP, Solomon PR, Thangaraj B, Raj SP, Solomon PR, et al. Fabaceae. Biodiesel from Flowering Plants. 2022:291-363.
[Crossref] [Google Scholar]
- Çiftçi S, Suna GÜ. Functional components of peanuts (Arachis hypogaea L.) and health benefits: A review. Future foods. 2022;5:100140.
[Crossref] [Google Scholar]
- Parilli-Moser I, Domínguez-López I, Trius-Soler M, Castellví M, Bosch B, et al. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin Nutr. 2021;40(11):5556-5567.
[Crossref] [Google Scholar] [PubMed]
- Cook BG, Pengelly BC, Brown SD, Donnelly JL, Eagles DA, et al. Tropical Forages: An interactive selection tool. 2005.
[Google Scholar]
- Jamil N, Zairi MN, Nasim NA, Pa'ee F. Influences of environmental conditions to phytoconstituents in Clitoria ternatea (butterfly pea flower)-A review. J Sci Technol. 2018;10(2).
[Google Scholar]
- Damodaran T, Cheah PS, Murugaiyah V, Hassan Z. The nootropic and anticholinesterase activities of Clitoria ternatea Linn. root extract: Potential treatment for cognitive decline. Neurochem Int. 2020;139:104785.
[Crossref] [Google Scholar] [PubMed]
- Kongkaew C, Scholfield NC, Dhippayom T, Dilokthornsakul P, Saokaew S, et al. Efficacy and safety of Pueraria candollei var. mirifica (Airy Shaw & Suvat.) Niyomdham for menopausal women: A systematic review of clinical trials and the way forward. J Ethnopharmacol. 2018;216:162-174.
[Crossref] [Google Scholar] [PubMed]
- Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front Med. 2012;6(1):8-21.
[Crossref] [Google Scholar] [PubMed]
- Cherdshewasart W, Kitsamai Y, Malaivijitnond S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J Reprod Dev. 2007;53(2):385-393.
[Crossref] [Google Scholar] [PubMed]
- Chansakaow S, Ishikawa T, Seki H, Sekine K, Okada M, et al. Identification of Deoxymiroestrol as the Actual Rejuvenating Principle of “Kwao Keur”, Pueraria mirifica. The Known Miroestrol May Be an Artifact. J Nat Prod. 2000;63(2):173-175. 1.
[Crossref] [Google Scholar] [PubMed]
- Passwater R. Pueraria mirifica: Just for menopause or the herb of the decade? Part I, Whole Foods Magazine. 2007.
[Google Scholar]
- Chulikhit Y, Sukhano W, Daodee S, Putalun W, Wongpradit R, et al. Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on ovariectomy-induced cognitive impairment and oxidative stress in the mouse brain. Molecules. 2021;26(11):3442.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Deng M. Identification and Control of Common Weeds: Volume 2. Dordrecht: Springer Netherlands; 2017.
[Crossref] [Google Scholar]
- Wien HC. Plant resources of South-East Asia. No. 8. vegetables: JS Siemonsma and Kasem Paluek (Editors), Pudoc, Wageningen, 1993, 412 pp., ISBN 90-220-1058-9. Sci Hortic. 1995;62(3):202-203.
[Crossref] [Google Scholar]
- Mandey JS, Soetanto H, Sjofjan O, Tulung B. Genetics characterization, nutritional and phytochemicals potential of gedi leaves (Abelmoschus manihot (L.) Medik) growing in the North Sulawesi of Indonesia as a candidate of poultry feed. Res J Biol. 2014;4(2):1276-1286.
[Google Scholar]
- Luan F, Wu Q, Yang Y, Lv H, Liu D, et al. Traditional uses, chemical constituents, biological properties, clinical settings, and toxicities of Abelmoschus manihot L.: A comprehensive review. Front Pharmacol. 2020;11:1068.
[Crossref] [Google Scholar] [PubMed]
- Zhou HR, Wu JR, Bei L, Wang BX, Xu H, et al. Hydroalcoholic extract from Abelmoschus manihot (Linn.) Medicus flower reverses sleep deprivation-evoked learning and memory deficit. Food Funct. 2020;11(10):8978-8986.
[Crossref] [Google Scholar] [PubMed]
- Orwa C. Agroforestree Database: A tree reference and selection guide, version 4.0. 2009.
[Google Scholar]
- Khare CP. Indian Medicinal Plants. 2007.
[Crossref] [Google Scholar]
- Rastogi RM. Compendium of Indian medicinal plants. Central Drug Research Institute, Lucknow, India. 1990;1:388-389.
[Google Scholar]
- Kumar SP, Bhushan MS, Trishna D, Arun M. Phytochemical investigation and antimicrobial activity of stem bark extracts of Thespesia populnea. Int J Pharm Res. 2010;1(1):24-29.
[Google Scholar]
- Puckhaber LS, Stipanovic RD. Thespesenone and dehydrooxoperezinone-6-methyl ether, new sesquiterpene quinones from Thespesia populnea. J Nat Prod. 2004;67(9):1571-1573.
[Google Scholar] [PubMed]
- Vasudevan M, Parle M. Pharmacological actions of Thespesia populnea relevant to Alzheimer's disease. Phytomedicine. 2006;13(9-10):677-687.
[Crossref] [Google Scholar] [PubMed]
- Plants of the World Online. The Royal Botanic Gardens. 2024.
- Zumbroich TJ. The origin and diffusion of betel chewing: A synthesis of evidence from South Asia, SoutheastAsia and beyond. E- Indian J Med. 2008;1(3):87.
[Google Scholar]
- Orwa C. Agroforestree Database: Areca catechu Arecaceae L, version 4.0. 2009.
- Staples GW, Bevacqua RF. Areca catechu (betel nut palm). Species profiles for Pacific Island agroforestry. 2006;1(13):1-9.
[Google Scholar]
- Sun H, Yu W, Li H, Hu X, Wang X. Bioactive Components of Areca Nut: An Overview of Their Positive Impacts Targeting Different Organs. Nutrients. 2024;16(5):695.
[Crossref] [Google Scholar] [PubMed]
- Joshi M, Gaonkar K, Mangoankar S, Satarkar S. Pharmacological investigation of Areca catechu extracts for evaluation of learning, memory and behavior in rats. Int Curr Pharm J. 2012;1(6):128-132.
[Google Scholar]
- Bhat SK, Ashwin D, Mythri S, Bhat S. Arecanut (Areca catechu L) decreases Alzheimer’s disease symptoms: Compilation of research works. J Med Plants Stud. 2017;5(5):4-9.
[Google Scholar]
- Sitaram N, Weingartner H, Gillin JC. Human serial learning: Enhancement with arecholine and choline impairment with scopolamine. Science. 1978;201(4352):274-276.
[Crossref] [Google Scholar] [PubMed]
- Christie JE, Shering A, Ferguson J, Glen AI. Physostigmine and arecoline: Effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry. 1981;138(1):46-50.
[Crossref] [Google Scholar] [PubMed]
- Prasansuklab A, Brimson JM, Tencomnao T. Potential Thai medicinal plants for neurodegenerative diseases: A review focusing on the anti-glutamate toxicity effect. J Tradit Complement Med. 2020 ;10(3):301-308.
[Crossref] [Google Scholar] [PubMed]
- GK S, MS Bharath M. Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(1):33-49.
[Crossref] [Google Scholar] [PubMed]
- Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive effect of nootropic drug Brahmi (Bacopa monnieri) in Alzheimer's disease. Ann Neurosci. 2017;24(2):111-22.
[Crossref] [Google Scholar] [PubMed]
- Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16(4):313-326.
[Crossref] [Google Scholar] [PubMed]
- Pase MP, Kean J, Sarris J, Neale C, Scholey AB, et al. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012;18(7):647-652.
[Crossref] [Google Scholar] [PubMed]
- Fatima U, Roy S, Ahmad S, Al-Keridis LA, Alshammari N, et al. Investigating neuroprotective roles of Bacopa monnieri extracts: Mechanistic insights and therapeutic implications. Biomed Pharmacother. 2022;153:113469.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Peng L, Zhang WD, Kong DY. Effect of triterpenoid saponins from Bacopa monniera on scopolamine-induced memory impairment in mice. Planta Med. 2009;75(06):568-574.
[Crossref] [Google Scholar] [PubMed]
- Njan AA, Adenuga FO, Ajayi AM, Sotunde O, Ologe MO, et al. Neuroprotective and memory-enhancing effects of methanolic leaf extract of Peristrophe bicalyculata in rat model of type 2 diabetes mellitus. Heliyon. 2020;6(5):e04011.
[Crossref] [Google Scholar] [PubMed]
- Brimson JM, Brimson SJ, Brimson CA, Rakkhitawatthana V, Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: Possible active compounds include lupeol, stigmasterol and β-sitosterol. Int J Mol Sci. 2012;13(4):5074-5097.
[Crossref] [Google Scholar] [PubMed]
- Esfandiari E, Ghanadian M, Rashidi B, Mokhtarian A, Vatankhah AM. The effects of Acorus calamus L. in preventing memory loss, anxiety, and oxidative stress on lipopolysaccharide-induced neuroinflammation rat models. Int J Prev Med. 2018;9(1):85.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acorus calamus: Scientific validation of ayurvedic tradition from natural resources. Pharm Biol. 2007;45(8):651-666.
[Crossref] [Google Scholar]
- Shin JW, Cheong YJ, Koo YM, Kim S, Noh CK, et al. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomol Ther (Seoul). 2014;22(1):17-26.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhang XL, Huang YR, Zheng YY, Zheng GQ, et al. Extracts or active components from Acorus gramineusaiton for cognitive function impairment: Preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2020;2020(1):6752876.
[Crossref] [Google Scholar] [PubMed]
- Cho N, Lee KY, Huh J, Choi JH, Yang H, et al. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem Toxicol. 2013;58:355-361.
[Crossref] [Google Scholar] [PubMed]
- Asuquo OR, Udonwa UN, Eluwa MA, Ekanem TB. Effects of Spondias mombin leaf extract on the cytoarchitecture of the cerebal cortex and on learning and memory in Wistar Rats. Int J Sci Res. 2013;2(9):5-8.
[Google Scholar]
- Porwal M, Kumar A. Neuroprotective effect of Annona squamosa & (-) Anonaine in decreased GABA receptor of epileptic rats. J Appl Pharm Sci. 2015;5(1):018-023.
[Crossref] [Google Scholar]
- Soumyanath A, Zhong YP, Henson E, Wadsworth T, Bishop J, et al. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer′ s Disease: Investigation of a possible mechanism of action. Int J Alzheimers Dis. 2012;2012(1):381974.
[Crossref] [Google Scholar] [PubMed]
- Pham HN, Vuong QV, Bowyer MC, Scarlett CJ. Phytochemicals derived from Catharanthus roseus and their health benefits. Technologies. 2020;8(4):80.
[Crossref] [Google Scholar]
- Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, et al. Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. J Ethnopharmacol. 2007;110(1):61-68.
[Crossref] [Google Scholar] [PubMed]
- Yamauchi Y, Ge YW, Yoshimatsu K, Komatsu K, Kuboyama T, et al. Memory enhancement by oral administration of extract of Eleutherococcus senticosus leaves and active compounds transferred in the brain. Nutrients. 2019;11(5):1142.
[Crossref] [Google Scholar] [PubMed]
- Kim CJ, Ryu HY, Lee S, Lee HJ, Chun YS, et al. Neuroprotective effect and antioxidant potency of fermented cultured wild ginseng root extracts of Panax ginseng CA meyer in mice. Molecules. 2021;26(10):3001.
[Crossref] [Google Scholar] [PubMed]
- Sharma K, Bhatnagar M, Kulkarni SK. Effect of Convolvulus pluricaulis Choisy. and Asparagus racemosus willd on learning and memory in young and old mice: A comparative evaluation. Indian J Exp Biol. 2010;48(5):479-485.
[Google Scholar] [PubMed]
- Akbaba E, Hassan S, Mohammed Sur T, Bagci E. Memory enhancing, anxiolytic and antidepressant effects of Achillea biebersteinii (Asteraceae) essential oil on scopolamine-induced rats. J Essent Oil-Bear Plants. 2018;21(3):825-839.
[Crossref] [Google Scholar]
- Cho N, Lee HK, Jeon BJ, Kim HW, Kim HP, et al. The effects of Betula platyphylla bark on amyloid beta-induced learning and memory impairment in mice. Food Chem Toxicol. 2014;74:156-163.
[Crossref] [Google Scholar] [PubMed]
- Sumanth M, Yashashwini YC. Evaluation of learning and memory enhancing activity of Commiphora caudata leaves in rats. World J Pharm Res. 2016;5(5):1223-1236.
[Google Scholar]
- Bhandari R, Gyawali S, Aryal N, Gaire D, Paudyal K, et al. Evaluation of Phytochemical, Antioxidant, and Memoryâ?Enhancing Activity of Garuga pinnata Roxb. Bark and Bryophyllum pinnatum (Lam) Oken. Leaves. Scientific World Journal. 2021;2021(1):6649574.
[Crossref] [Google Scholar] [PubMed]
- Kim JI, Jeon SG, Kim KA, Kim JJ, Song EJ, et al. Platycodon grandiflorus root extract improves learning and memory by enhancing synaptogenesis in mice hippocampus. Nutrients. 2017;9(7):794.
[Crossref] [Google Scholar] [PubMed]
- Cho J, Kang JS, Long PH, Jing J, Back Y, et al. Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res. 2003;26:821-825.
[Crossref] [Google Scholar] [PubMed]
- Wahid S, Alqahtani A, Khan RA. Cucurbita maxima seeds reduce anxiety and depression and improve memory. Behav Neurol. 2023;2023(1):7509937.
[Crossref] [Google Scholar] [PubMed]
- Patro G, Bhattamisra SK, Mohanty BK. Effects of Mimosa pudica L. leaves extract on anxiety, depression and memory. Avicenna J Phytomed. 2016;6(6):696-710.
[Google Scholar] [PubMed]
- Beppe GJ, Dongmo AB, Foyet HS, Tsabang N, Olteanu Z, et al. Memory-enhancing activities of the aqueous extract of Albizia adianthifolia leaves in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. BMC Complement Altern Med. 2014;14:142.
[Crossref] [Google Scholar] [PubMed]
- Kim DH, Yoon BH, Kim YW, Lee S, Shin BY, et al. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J Pharmacol Sci. 2007;105(1):82-93.
[Crossref] [Google Scholar] [PubMed]
- Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004;91(2-3):361-365.
[Crossref] [Google Scholar] [PubMed]
- Moriasi GA, Ireri AM, Ngugi MP. In Vivo Cognitiveâ?Enhancing, Ex Vivo Malondialdehydeâ?Lowering Activities and Phytochemical Profiles of Aqueous and Methanolic Stem Bark Extracts of Piliostigma thonningii (Schum.). Int J Alzheimers Dis. 2020;2020(1):1367075.
[Crossref] [Google Scholar] [PubMed]
- Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res Pharm Sci. 2014;9(2):107-114.
[Google Scholar] [PubMed]
- Park SJ, Kim DH, Lee IK, Jung WY, Park DH, et al. The ameliorating effect of the extract of the flower of Prunella vulgaris var. lilacina on drug-induced memory impairments in mice. Food Chem Toxicol. 2010;48(6):1671-1676.
[Crossref] [Google Scholar] [PubMed]
- Ozarowski M, Mikolajczak PL, Piasecka A, Kujawski R, Bartkowiak-Wieczorek J, et al. Effect of Salvia miltiorrhiza root extract on brain acetylcholinesterase and butyrylcholinesterase activities, their mRNA levels and memory evaluation in rats. Physiol Behav. 2017;173:223-230.
[Crossref] [Google Scholar] [PubMed]
- Moss L, Rouse M, Wesnes KA, Moss M. Differential effects of the aromas of Salvia species on memory and mood. Hum Psychopharmacol. 2010;25(5):388-396.
[Crossref] [Google Scholar] [PubMed]
- Heo H, Shin Y, Cho W, Choi Y, Kim H, et al. Memory improvement in ibotenic acid induced model rats by extracts of Scutellaria baicalensis. J Ethnopharmacol. 2009;122(1):20-27.
[Crossref] [Google Scholar] [PubMed]
- Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer’s type. J Basic Clin Physiol Pharmacol. 2015;26(3):275-285.
[Crossref] [Google Scholar] [PubMed]
- Rajesh V, Riju T, Venkatesh S, Babu G. Memory enhancing activity of Lawsonia inermis Linn. leaves against scopolamine induced memory impairment in Swiss albino mice. Orient Pharm Exp Med. 2017;17(2):127-142.
[Crossref] [Google Scholar]
- Nandhini S, Vadivu R, Jayshree N. Memory strengthening activity on seeds of Abelmoschus moschatus. IJRPC. 2014;4(2).
[Google Scholar]
- Kulkarni PD, Ghaisas MM, Chivate ND, Sankpal PS. Memory enhancing activity of Cissampelos pariera in mice. Int J Pharm Sci. 2011;3(2):206-211.
[Google Scholar]
- Wachiryah TA, Hathaipat L. Enhancing effect of Tiliacora triandra leaves extract on spatial learning, memory and learning flexibility as well as hippocampal choline acetyltransferase activity in mice. Avicenna J Phytomed. 2018;8(4):380-388.
[Google Scholar] [PubMed]
- Ahmed F, Chandra JN, Manjunath S. Acetylcholine and memory-enhancing activity of Ficus racemosa bark. Pharmacognosy research. 2011;3(4):246-249.
[Crossref] [Google Scholar] [PubMed]
- Parle M, Dhingra D, Kulkarni SK. Improvement of mouse memory by Myristica fragrans seeds. J Med Food. 2004;7(2):157-161.
[Crossref] [Google Scholar] [PubMed]
- Sriraksa N, Kaewwongse M, Phachonpai W, Hawiset T. Effects of lemongrass (Cymbopogon citratus) essential oil inhalation on cognitive performance and mood in healthy women.
- Thai Pharmaceutical and Health Science Journal. 2018;13(2):80-88.
[Google Scholar]
- Minami A, Matsushita H, Horii Y, Ieno D, Matsuda Y, et al. Improvement of depression-like behavior and memory impairment with the ethanol extract of Pleurotus eryngii in ovariectomized rats. Biol Pharm Bull. 2013;36(12):1990-1995.
[Crossref] [Google Scholar] [PubMed]
- Liang CH, Huang PC, Mau JL, Chiang SS. Effect of the king oyster culinary-medicinal mushroom Pleurotus eryngii (Agaricomycetes) basidiocarps powder to ameliorate memory and learning deficit in ability in Aβ-induced Alzheimer's disease C57BL/6J mice model. Int J Med Mushrooms. 2020;22(2).
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Ma C, Li BM, Sun C. Polygala japonica Houtt. reverses depression-like behavior and restores reduced hippocampal neurogenesis in chronic stress mice. Biomed Pharmacother. 2018;99:986-996.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Liu Y, Wang L, Liu X, Chang Q, et al. Memoryâ?enhancing effects of the crude extract of Polygala tenuifolia on aged mice. Evid Based Complement Alternat Med. 2014;2014(1):392324.
[Crossref] [Google Scholar] [PubMed]
- Norouzi F, Hosseini M, Abareshi A, Beheshti F, Khazaei M, et al. Memory enhancing effect of Nigella sativa hydro-alcoholic extract on lipopolysaccharide-induced memory impairment in rats. Drug Chem Toxicol. 2019;42(3).
[Crossref] [Google Scholar] [PubMed]
- Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, et al. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol. 2013;145(2):431-441.
[Crossref] [Google Scholar] [PubMed]
- Mikulska P, Malinowska M, Ignacyk M, Szustowski P, Nowak J, et al. Ashwagandha (Withania somnifera)-Current research on the health-promoting activities: A narrative review. Pharmaceutics. 2023;15(4):1057.
[Crossref] [Google Scholar] [PubMed]
- Zhao Z, Li Y, Chen H, Huang L, Zhao F, et al. Xylaria nigripes mitigates spatial memory impairment induced by rapid eye movement sleep deprivation. Int J Clin Exp Med. 2014;7(2):356-362.
[Google Scholar] [PubMed]
- Muralidharan P, Kumar VR, Balamurugan G. Protective effect of Morinda citrifolia fruits on βâ?amyloid (25–35) induced cognitive dysfunction in mice: An experimental and biochemical study. Phytother Res. 2010;24(2):252-258.
[Crossref] [Google Scholar] [PubMed]
Citation: Potential of Natural Substance Usage in Southeast Asia for Memory Enhancement: A Review ASEAN Journal of Psychiatry, Vol. 25 (7) September, 2024; 1-18.