NEUROPSYCHIATRIC ISSUES IN PARKINSON DISEASE (PD) PATIENTS: CLINICAL FEATURES AND GENETICS PREDISPOSITION
Department of Medicine,, Queen Elizabeth Hospital, Hong Kong, China
*Corresponding Author:
Germaine Hiu-Fai Chan, Department of Medicine,,
Queen Elizabeth Hospital, Hong Kong,
China,
Email: Chf862@ha.org.hk
Received: 02-Mar-2023, Manuscript No. AJOPY-22-90592;
Editor assigned: 06-Mar-2023, Pre QC No. AJOPY-22-90592 (PQ);
Reviewed: 20-Mar-2023, QC No. AJOPY-22-90592;
Revised: 27-Mar-2023, Manuscript No. AJOPY-22-90592 (R);
Published:
07-Nov-2023, DOI: 10.54615/2231-7805.S3.002
Editorial Note
Neuropsychiatric symptoms are common and bothersome to both the Parkinson Disease (PD) patients and their caregivers. They can be classified into four groups: affective disorder, psychosis, cognitive dysfunction and impulse control disorder. In this mini-review, we will offer an overview of neuropsychiatric symptoms in PD patients and discuss if there is a genetic predisposition to these psychiatric symptoms.
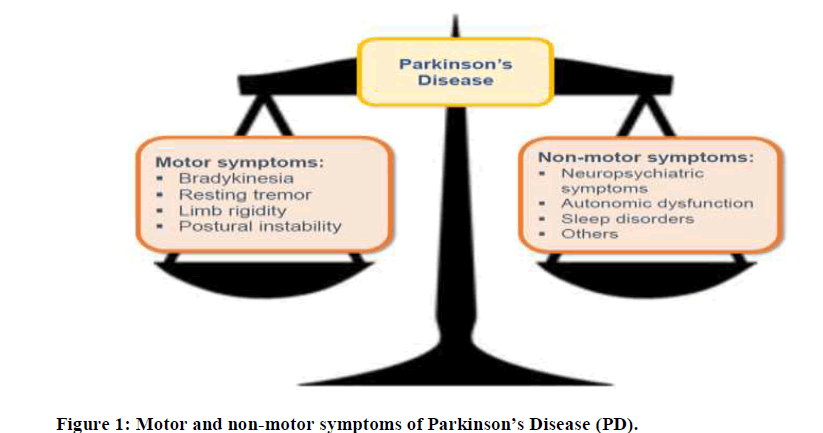
Parkinson’s Disease (PD) is the second commonest neurodegenerative disease in the world affecting both the motor and non-motor domains (Figure 1). In general, it affects 1% of the population above 60 years of age [1]. Even though motor symptoms remain the cardinal features of the disease, it is known that PD patients have a wide spectrum of non-motor symptoms.
Barone have reported that almost all the PD patients have at least one non-motor symptoms, with psychiatric symptoms as the most frequent non-motor symptom (67%) [2]. Similar findings were described in the Australian group. [3,4] According to the 15-year Sydney multicentre Study of PD, cognitive decline was observed in 84% of PD patients whereas depression and hallucination were seen in half of the cohort [3]. In the 20-year follow-up study of the same cohort, visual hallucination and dementia were present in 74% and 83%, respectively [4]. Eventually, these neuropsychiatric symptoms lead to a reduction in the dopaminergic medication dosage, as well as, an addition of psychiatric medications (such as antipsychotics and cholinesterase inhibitors), and hence attribute to a decline in motor function, loss of independence, institutionalization and mortality.
As discussed earlier, neuropsychiatric symptoms are common and bothersome to PD patients. Moreover, they can be seen in all stages of the disease. At the prodromal stage, some patients may experience mood disorders such as apathy.
On the contrary, advanced PD patients can develop psychosis and dementia. In general, neuropsychiatric symptoms in PD can be classified into 4 groups and will be discussed in detail.
1. Affective disorder
2. Psychosis
3. Cognitive dysfunction
4. Impulse control disorder
Affective disorder
Affective disorder is frequently encountered in PD patients, though it is often neglected by both the patients and health care professionals. It includes apathy, depression, and anxiety. In some cases, PD patients with mood disorders may develop suicidal ideas, or even commit suicide.
Reijnders, et al. reported that the prevalence rate of clinically significant depressive symptoms in PD was 35%, with a wide variation across studies, ranging from 2.7% to more than 90% [5]. A recent review conducted by a Chinese group showed similar findings and revealed that the prevalence of depression in PD was 38% [6]. Also, anxiety disorder was found in 25%-50% of PD patients [7- 9].
Moreover, depression and anxiety often overlaps, making the diagnosis difficult. In addition, mood symptoms can coexist with other motor symptoms in PD. The PROMS-PD Study Group from the UK suggested an increased risk of anxiety in patients with motor fluctuations, especially with a young age of onset [10]. On the other hand, depression was strongly associated with freezing of gait and axial motor symptoms [10].
The degeneration of the extranigral pathways, which results in a loss of noradrenergic, serotonergic and cholinergic neurons, may account for the co-occurrence of levodopa refractory gait dysfunction and mood disorders. Furthermore, mood disorders such as depression and anxiety were found to be associated with poorer quality of life in PD patients [11]. In particular, patients with depression were reported to have longer disease duration and a poorer motor function, which may account for a decline in quality of life [11].
Psychosis
PD psychosis is defined as the presence of at least one of the characteristic symptoms, namely illusions, false sense of presence, hallucinations or delusions, which occurred after disease onset. These symptoms must be recurrent or continuous for one month. Besides, there are no alternative diagnoses that can reasonably account for the symptom.
Furthermore, these symptoms may be associated with loss of insight, dementia, or use of antiparkinsonian treatment [12]. Psychosis is prevalent and bothersome in PD patients and so it can cause poorer quality of life, increased caregivers’ stress, institutionalization, and mortality.
In a 12-year prospective longitudinal study, 60% of PD patients were found to develop either delusions or hallucinations by the end of the study [13]. Moreover, the presence of delusions or hallucinations was reported to be significantly associated with nursing home placement [14].
Likewise, PD patients with psychosis were found to have a 70% increased risk of mortality, as compared with those without psychosis [15]. In general, PD psychosis can be divided into two groups: early PD psychosis symptoms and late PD psychosis symptoms.
In early PD, patients may experience passage hallucinations (i.e. seeing a non-existing person or object passing in the peripheral visual field), presence hallucinations (i.e. feeling that somebody is in the proximity), and illusions.
With disease progression, patients develop formed visual hallucinations of people, animals, or objects with preservation of insight. At a late stage of PD, delusions and multimodality hallucinations will occur with loss of insight and cognitive decline [16].
Cognitive dysfunction
Dementia associated with PD is defined as an insidious, slowly progressive dementia syndrome, which develops in an established PD patient [17]. He usually has both cognitive and behavioural symptoms. Classically, executive dysfunction and visuospatial impairment are the cardinal features of cognitive profile in PD dementia.
For instance, the patient may find it difficult to follow the instructions of taking medications. Also, a patient with PD dementia often suffers from impaired attention, poor recall, and short-term memory. His long-term memory, however, is usually preserved. In contrast, behavioural problems are prevalent in these patients, particularly apathy, visual hallucination, and psychosis [18].
PD dementia is common, with a point prevalence rate ranging from 22%-48% [19,20]. With increasing age and longer disease duration, the prevalence rate of PD dementia further increases. It has been reported that 15 years after disease onset, almost half of PD patients have dementia [3].
It is crucial to identify cognitive impairment and dementia in PD patients because this can affect the choice of treatment. For example, we may need to cut down the dosage of anti-cholinergic agents in the PD patients with cognitive impairment.
On the contrary, the presence of severe dementia can preclude a patient from implantation of deep brain stimulation. Furthermore, with the executive dysfunction and visuo-spatial impairment in patients with PD dementia, driving can be dangerous to both the patients and pedestrians [21].
Impulse control disorder
Impulse Control Disorders (ICDs) are known as a group of disorders which is characterised by a failure to resist an impulse, drive, or temptation to perform an act that is harmful to the individual or to others. It is frequently seen in PD patients treated with dopaminergic agents, especially levodopa and dopamine agonists [22]. According to the DOMINION study, ICDs are found in 13.6% of PD patients [23].
In brief, ICDs are addictive behaviours and include pathological gambling, hypersexuality, compulsive shopping, binge eating, punding and compulsive medication use [22]. In addition, PD patients with ICDs experience more neuropsychiatric problems, such as depressive and anxiety issues, obsessivecompulsive symptoms, novelty seeking and impulsivity behaviours.
They are also found to be more disabled. Those with multiple ICDs are reported to have more dyskinesia [24]. Studies showed that young age, male sex and history of smoking, together with use of levodopa and dopamine agonists (especially with a high dosage), are risk factors of ICDs in PD patients [25].
Therefore, it may be worthwhile to watch out for these impulse control behaviours in young, male PD patients taking dopamine agonists because these behavioural problems can significantly affect their economy, social and family relationship.
Genetic predisposition of neuropsychiatric problems in PD: A fact or a myth
To date, there is no literature comparing the overall prevalence of neuropsychiatric symptoms in idiopathic PD and in monogenic parkinsonism. Nevertheless, for some types of monogenic parkinsonism, the patients are reported to be predisposed to neuropsychiatric problems, especially cognitive impairment.
For example, patients with SNCA and GBA mutations were at a higher risk of dementia while those with PRKN mutation seldom developed cognitive impairment. Neuropsychiatric symptoms are associated with poor quality of life and institutionalization in PD patients. They can also increase caregivers’ burden.
The genetic risk factors of each neuropsychiatric disorder in PD are shown in Table 1.
| Author |
Study design |
Number of subjects |
Results |
| Affective disorder |
| Cong, et al. [6] |
Systematic review |
38304 |
PD patients with depression were more likely to have the GBA1 L444P mutation. |
| An association between PD patients with depression and LRRK2 G2019S mutation was not reported. |
| An association between PD patients with depression and SNCA rs356219 polymorphism was not observed. |
| Beavan, et al.[26] |
Prospective cohort study |
90 |
PD patients with GBA mutation were found to have higher depression scores. |
| Psychosis |
| RadojeviÄ?, Branislava, et al. [27] |
Cross-sectional study |
234 |
GG homozygotes of rs2734849 ANKK1 mutation were found to be an independent predictor of PD psychosis. |
| Oeda, et al. [28] |
Multi-centre retrospective cohort study |
224 |
PD patients with GBA mutation were found to develop psychosis significantly earlier than those without mutation. |
| Fuente-Fernández, et al. [29] |
Cross-sectional study |
17 |
The APOE 4 allele was significantly associated with the development of visual hallucination in non-demented PD patients. |
| Cognitive dysfunction |
| Campelo, et al. [30] |
Case control study |
PD patients: 105 |
SNCA G-rs356219 single nucleotide polymorphism was associated with an increased risk of cognitive impairment in PD patients. |
| Control: 101 |
| Tan, et al. [31] |
Prospective cohort study |
3364 |
APOE 4 variant, rs429358, was significantly associated with cognitive progression in PD. |
| Liu, et al. [32] |
Longitudinal study |
3821 |
GBA and APOE mutations were reported to be associated with cognitive progression in PD. |
| Alcalay, et al. [33] |
Case control study |
93 |
Early onset PD patients with GBA mutation were found to have poorer performance on the Mini-Mental State Examination, especially on the memory and visuospatial domains. |
| GBA mutation: 33 |
| No GBA mutation: 60 |
| Cilia, et al. [34] |
Prospective longitudinal study |
2843 |
PD patients with GBA mutation were found to have a greater risk of dementia. |
| Impulse control disorder |
| Jesús, et al. [35] |
Cross-sectional study |
353 |
DOPA decarboxylase gene (DDC), rs1451375, may modulate the risk of ICDs in PD patients. |
| Lee, et al. [36] |
Cross-sectional study |
404 |
The T allele of HTR2A gene was associated with impulse control behaviours in PD. |
| Lee, et al. [37] |
Cross-sectional study |
404 |
Carriers of either AA genotype of DRD3 or CC genotype of GRIN2B were associated with the occurrence of impulse control behaviours in PD. |
Table 1: Genetic risk factors of neuropsychiatric disorders in PD.
Therefore, it is crucial to watch out for these symptoms and offer timely treatment, especially in the PD patients who are known to have risk factors.
References
- De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. The Lancet Neurology. 2006; 5(6): 525-535.
[Crossref] [Google Scholar] [PubMed]
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. PRIAMO study group. The PRIAMO study: A multicenter assessment of
- Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Movement Disorders. 2005; 20(2):190-199.
[Crossref] [Google Scholar] [PubMed]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Movement Disorders. 2008; 23(6):837-844.
[Crossref] [Google Scholar] [PubMed]
- Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Movement Disorders. 2008; 23(2):183-189.
[Crossref] [Google Scholar] [PubMed]
- Cong S, Xiang C, Zhang S, Zhang T, Wang H, Cong S. Prevalence and clinical aspects of depression in Parkinson's disease: A systematic review and meta-analysis of 129 studies. Neuroscience & Biobehavioral Reviews. 2022; 141: 104749.
[Crossref] [Google Scholar] [PubMed]
- Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SA, Grill S, et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson's disease. Movement Disorders. 2009; 24(9):1333-1338.
[Crossref] [Google Scholar] [PubMed]
- Dissanayaka NN, Sellbach A, Matheson S, O'Sullivan JD, Silburn PA, Byrne GJ, et al. Anxiety disorders in Parkinson's disease: prevalence and risk factors. Movement Disorders. 2010; 25(7):838-845.
[Crossref] [Google Scholar] [PubMed]
- Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson's disease: A systematic review and meta-analysis. Movement Disorders. 2016; 31(8):1125-1133.
[Crossref] [Google Scholar] [PubMed]
- Burn DJ, Landau S, Hindle JV, Samuel M, Wilson KC, Hurt CS, et al. PROMS-PD Study Group. Parkinson's disease motor subtypes and mood. Movement Disorders. 2012; 27(3):379-386.
[Crossref] [Google Scholar] [PubMed]
- Su W, Liu H, Jiang Y, Li S, Jin Y, Yan C, et al. Correlation between depression and quality of life in patients with Parkinson's disease. Clinical Neurology and Neurosurgery. 2021; 202:106523.
[Crossref] [Google Scholar] [PubMed]
- Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Movement Disorders. 2007; 22(8):1061-1068.
[Crossref] [Google Scholar] [PubMed]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D, et al. A 12-year population-based study of psychosis in Parkinson disease. Archives of Neurology. 2010; 67(8):996-1001.
[Crossref] [Google Scholar] [PubMed]
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology. 1993; 43(11):2227-2229.
[Crossref] [Google Scholar] [PubMed]
- Stang CD, Mullan AF, Camerucci E, Hajeb M, Turcano P, Martin P, et al. Incidence, Prevalence, and Mortality of Psychosis Associated with Parkinson's Disease (1991-2010). Journal of Parkinsons Disease. 2022; 12(4):1319-1327.
[Crossref] [Google Scholar] [PubMed]
- Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, et al. The psychosis spectrum in Parkinson disease. Nature Reviews Neurology. 2017; 13(2):81-95.
[Crossref] [Google Scholar] [PubMed]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders. 2007; 22(12):1689-1707.
[Crossref] [Google Scholar] [PubMed]
- Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson's disease. Journal of Neurological Sciences. 2017; 374:26-31.
[Crossref] [Google Scholar] [PubMed]
- Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Movement Disorders. 2004; 19(9):1043-1049.
[Crossref] [Google Scholar] [PubMed]
- De Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Archives of Neurology. 2005; 62(8):1265-1269.
[Crossref] [Google Scholar] [PubMed]
- Emre M, Ford PJ, Bilgiç B, Uç EY. Cognitive impairment and dementia in Parkinson's disease: practical issues and management. Movement Disorders. 2014; 29(5):663-672.
[Crossref] [Google Scholar] [PubMed]
- Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Archives of Neurology. 2007; 64(8):1089-1096.
[Crossref] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Archives of Neurology. 2010; 67(5):589-595.
[Crossref] [Google Scholar] [PubMed]
- Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Annals of Neurology. 2011; 69(6):986-996.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Luo W, Mo Y, Wei C, Tao R, Han M. Meta-analysis of related factors of impulse control disorders in patients with Parkinson's disease. Neuroscience Letters. 2019; 707:134313.
[Crossref] [Google Scholar] [PubMed]
- Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurology. 2015; 72(2):201-208.
[Crossref] [Google Scholar] [PubMed]
- Radojevic B, Dragaševic-Miškovic NT, Marjanovic A, Brankovic M, Dobricic V, Milovanovic A, et al. Clinical and Genetic Analysis of Psychosis in Parkinson's Disease. Journal of Parkinsons Disease. 2021; 11(4):1973-1980.
[Crossref] [Google Scholar] [PubMed]
- Oeda T, Umemura A, Mori Y, Tomita S, Kohsaka M, Park K, et al. Impact of glucocerebrosidase mutations on motor and nonmotor complications in Parkinson's disease. Neurobiology of Aging. 2015; 36(12):3306-3313.
[Crossref] [Google Scholar] [PubMed]
- De la Fuente-Fernández R, Núñez MA, López E. The apolipoprotein E epsilon 4 allele increases the risk of drug-induced hallucinations in Parkinson's disease. Clinical Neuropharmacology. 1999; 22(4):226-230.
[Google Scholar] [PubMed]
- Campêlo CLC, Cagni FC, de Siqueira Figueredo D, Oliveira LG Jr, Silva-Neto AB, Macêdo PT, et al. Variants in SNCA Gene Are Associated with Parkinson's Disease Risk and Cognitive Symptoms in a Brazilian Sample. Frontiers in Aging Neuroscience. 2017; 9:198.
[Crossref] [Google Scholar] [PubMed]
- Tan MMX, Lawton MA, Jabbari E, Reynolds RH, Iwaki H, Blauwendraat C, et al. Genome-Wide Association Studies of Cognitive and Motor Progression in Parkinson's Disease. Moving Disorders. 2021; 36(2):424-433.
[Crossref] [Google Scholar] [PubMed]
- Liu G, Peng J, Liao Z, Locascio JJ, Corvol JC, Zhu F, et al. Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson's disease. Nature Genetics. 2021; 53(6):787-793.
[Crossref] [Google Scholar] [PubMed]
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012; 78(18):1434-40.
[Crossref] [Google Scholar] [PubMed]
- Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson's disease: The mutation matters. Annals of Neurology. 2016; 80(5):662-673.
[Crossref] [Google Scholar] [PubMed]
- Jesús S, Periñán MT, Cortés C, Buiza-Rueda D, Macías-García D, Adarmes A, et al. Integrating genetic and clinical data to predict impulse control disorders in Parkinson's disease. European Journal of Neurology. 2021; 28(2):459-468.
[Crossref] [Google Scholar] [PubMed]
- Lee JY, Jeon BS, Kim HJ, Park SS. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson's disease. Parkinsonism & Related Disorders. 2012; 18(1):76-78.
[Crossref] [Google Scholar] [PubMed]
- Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson's disease. Movement Disorders. 2009; 24(12):1803-1810.
[Crossref] [Google Scholar] [PubMed]