Introduction
Music has often been used as an effective treatment for psychological diseases. The neuroscientific approach proved music exerted a strong influence on the complex neurobiology procedures of the brain, reducing symptoms of mental disorders including depression, anxiety, autism, schizophrenia and dementia, etc [1]. It has been reported that music was able to prevent depression and anxiety that are induced by chronic unpredictable mild stress by restoring the levels of BDNF protein [2]. Notably, music therapy is found to alleviate both physical pain and psychological pain for terminally ill patients, while music therapy combined with standardized treatments gives better outcomes in improving depression, anxiety, schizophrenia, Obsessive-Compulsive Disorder (OCD) in patients [3-7].
Nevertheless, most of the previous studies specifically focus on producing soothing and comforting effects by music. However, different music tracks give different levels of relaxation, with loud beats of drums detrimental to the level of relaxation [8]. There are doubts about whether a Articledifferent musical composition will have the exact opposite effects on a human’s emotional state.
Hip-hop music and heavy metal music are two controversial types of music that appeal to teenagers more than other age groups [9]. Studies show a general correlation between antisocial behaviors and listening to rap, which includes hip-hop and heavy metal music [10]. Rap music including hip-hop is found to be associated with deviant behaviors (violence, theft, street gangs, mild drug use and hard drug use) in adolescents [11]. Relationship is found between heavy metal music and depression, delinquency, risk-taking and drug-taking behaviors, suicidal thinking and deliberate self-harm, despite that the underlying mechanisms is unknown due to influences of other factors such as family situations [12-14]. Indeed, heavy metal’s usual hopeless theme might be the main cause of it usually becoming the victim to be blamed for teenager’s psychological health [12]. Still, other studies claim that the miserable themes prevalent in heavy metal music provide an emotional outlet for adolescents, improving their well-being and fans of heavy metal exhibit no anxiety-like or depressive behaviors [15].
Therefore, it is reasonable to doubt the validity
of the correlation between heavy metal music and
mental disorders [9].
Moreover, significant associations were found
between noise annoyance and depression, anxiety
disorder and general mental health [16]. 4 weeks
of daily exposure to non-traumatic white noise is
found to have anxiogenic effects through elevating
the lateral amygdala’s neuronal activity [17].
Furthermore, the risk of depression was increased
by exposure to aircraft noises [18]. However, the
chronic effect of construction noise on anxiety or
depression was never deeply investigated in the
previous studies.
Teenage brains are characterized by a variety of
modulations, including a decrease in gray matter
accompanied by an increase in white matter,
a decrease in neuronal density and synaptic
pruning, a shift from diffuse to focal of cortical
activity, an increase in ventral striatum activity,
amygdala hyperactivation with relatively weaker
amygdala-hippocampus connectivity, etc and
most of which show a controversial correlation
with adolescent’s impulsive behaviors [19-24].
Some studies attribute teenage impulsive actions
to an imbalance of development between earlier
maturation of subcortical regions, specifically, the
striatum and less mature prefrontal cortex (one
indication of development is the increase in density
of dopamine receptors D1 and D2, which is said
to account for adolescent’s sensitivity to rewards)
[25]. Other studies explain that the remodeling of
the prefrontal cortex and the dopaminergic system
during adolescence leads to anticipations of more
abstract and distant types of rewards, which is also
more likely to be frustrated, causing adolescents
to be more vulnerable to depression [26,27].
Social stressors (including social instability and
social defeat) applied during adolescence have
been shown to promote anxious and depressive
behaviors in rodent models [28]. Both acute and
chronic stresses act via diverse neuronal circuits
to induce anxiety and depression, while the
early life environment greatly influences stressor
reactivity throughout the life span [29]. 3-weeks
of Chronic Mild Stress (CMS) protocol is shown
to induce depression through the observation of a
decrease in the sucrose preference and an increase
in the immobility time in the forced swim test and
shown to induce anxiety through the observation
of a reduction in open arm exploration in elevated
plus maze test [30-32]. Nonetheless, few studies have been made concerning acoustic stress during
adolescence.
Thus, heavy metal music, hip-hop music, or
construction noise were applied in our study to
evaluate the effects of those voice on emotional
state (mainly depression and anxiety) on teenage
mice. Therefore, 8-week mice as models for young
teenagers were used to measure how acoustic
stress stimuli may alter the composition and
development of the brain, which is then reflected
in the behavior. Several behavioral tests including
the Sucrose Preference Test (SPT), the Nest
Building (NB), the Open Field Test (OFT), the
Elevated Plus Maze (EPM), the Tail Suspension
Test (TST) and the Forced Swim Test (FST)
were performed to determine the anxiety and
depression level of rodent models. The underlying
mechanisms of those voice regulating depressive
or anxious behaviors were slightly investigated
by measuring the dopamine receptor, serotonin,
brain-derived neurotrophic factors and Trk B’s
(Tropomyosin receptor kinase B) level in the brain
of mice.
Materials and Methods
Experimental design
The study aims to investigate the impact of
long-term exposure to acoustic stimuli over one
week on mice in adolescence (8 weeks old). To
minimize variation in the parameters of interest,
mice were litter-matched, age-matched and sexmatched
in all animal trials. The chosen acoustic
stimuli are intended to focus on intense sounds that
may arouse anxiety-like or depressive emotions
after long and constant exposure. Two groups of
music, hip-hop and heavy metal and construction
noise were applied in the study. There were four
groups of mice, with ten mice per group. Each
of them was placed in identical chambers with
similar treatments except that three groups were
put under three distinct chronic sound stresses
from 9 am to 12 pm and from 2 pm to 5 pm every
day, giving them a total of six hours of acoustic
stimuli each day. The voice box is stuck to the top
of the box about 0.25 m from the mice. Every day,
at around 1 pm, each mouse is weighed and the
Sucrose Preference Test (SPT), the Nest Building
(NB), the Open Field Test (OFT), the Elevated
Plus Maze (EPM), the Tail Suspension Test (TST)
and the Forced Swim Test (FST) are conducted
to evaluate anxiety or depression-like behaviors
in mice. The frontal lobe and hippocampus were collected and the levels of protein expression
(dopamine receptor 1-type, (DR1); BDNF; Trk
B) and neurotransmitter (serotonin, 5-HT) were
estimated by Enzyme-Linked Immunosorbent
Assay (ELISA) and western blot assays.
Animal experiment
All animal studies were carried out in strict
conformity with the Nanjing University of
Chinese Medicine’s institutional ethical standards
on animal care. Every Specific Pathogen-Free
(SPF) animal was given sterile SPF pellet rodent
feed and sterile water. They were also kept in
standard environments, with a room temperature
of 22°C and a 12 hour light/dark cycle. Wild-
type C57BL/6 male mice (8 weeks old) were used. Experimental groups were divided into groups of WT mice without any intentional acoustic stimuli (WT, n=10), mice under hip-hop stimuli (HH, n=10), mice under heavy metal stimuli (HM, n=10) and mice under construction noise stimuli (CN, n=10).
Sound exposure
The music lists for hip-hop and heavy metal are
below and with an average of 3 min-4 min per
song and a total of about 30 min for all songs in
either hip-hop group or heavy metal group. The
mice were exposed with different sounds from 9
am to 12 pm and from 2 pm to 5 pm every day for
one week.
Body weight
The initial weights of mice were recorded and the
mice were weighted every day at 2 pm for one
week. The averages of the weights of mice were
calculated daily and the trends of the weights over
the week were graphed.
Sucrose Preference Test (SPT)
The sucrose preference test is conducted to
evaluate the sound exposure-induced anhedonia
in mice models [33]. The mice were given two
bottles, with one 100 mL of 5% sucrose solution
and one with an equivalent amount of water,
at the start of the trial. The water and sucrose
solution were provided on a 24 hour basis every
day. The residual volume of each bottle was
measured every day at around 2 pm for estimation
of the total volume of water and sucrose solution
consumed in each group. After the one-week trial,
the sucrose preference (%) for each group of mice
was calculated as the amount of sucrose solution
intake over the total liquid intake each day. Data consisting of six data corresponding to six days
were obtained for the sucrose preferences for mice
under four different sound exposures.
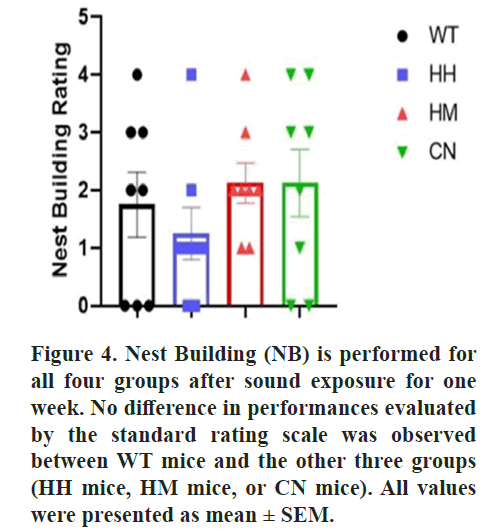
Nest Building (NB)
The nest-building behavioral test was performed
according to the previously published approach
[34]. Briefly, each mouse was individually placed
in an open chamber of 15 cm × 40 cm × 20 cm and
given available water and two pieces of cotton.
The mice were given a night time to build a nest
with the cotton, the results were photographed and
scored basing on a rudimentary scale of 4 [34].
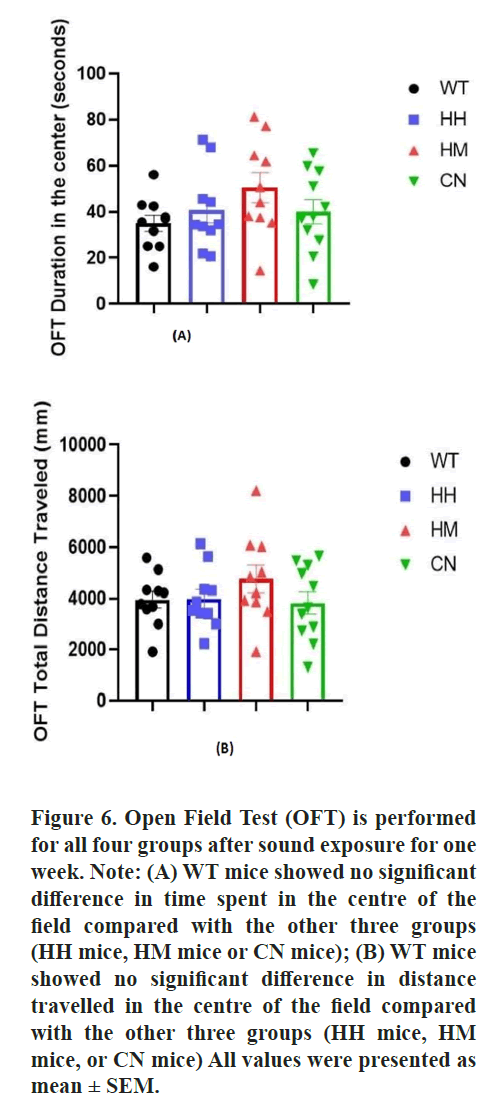
Open Field Test (OFT)
The open field test was performed according to
the previously published approach [35]. The mice
were placed in an open field chamber (length,
width and height: 25 cm × 25 cm × 40 cm) for 10
min briefly, the mice were initially placed in the
center and their movements were recorded by a
video tracking system. The open field is divided
into a central field and an outer field. The time
mice spent in both fields and the total distances
traveled for each mouse were recorded. After each
trial, the apparatus is cleaned with 70% ethanol
to remove smells that may interfere with the
following mice’s performances.
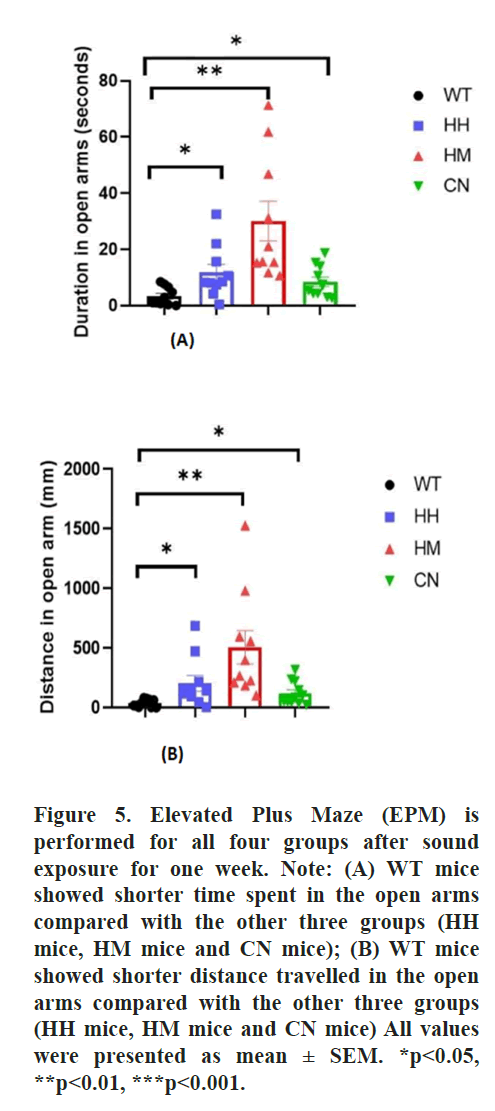
Elevated Plus Maze (EPM)
The elevated plus-maze and the testing procedure
were performed according to the previously
published approach [36]. The Elevated Plus Maze
(EPM) consisted of two open arms (50 cm × 10 cm)
and two closed arms (50 cm × 10 cm) elevated 50
cm above the ground. Briefly, mice were initially
placed in the center or the conjunction of the four
arms facing a closed arm and allowed to explore
for 5 min freely. A video tracking system tracked
their movements and recorded the time mice
spent in open and closed arms and the number of
entrances made to each arm. After each trial, the
apparatus is cleaned with 70% ethanol to remove
smells that may interfere with the following mice’s
performances.
Tail Suspension Test (TST)
The tail suspension test was performed according
to the previously published approach [37].
Mice were suspended by the tails about 50 cm
above the ground for 6 min. The mouse tail was
secured to the hook at the top by adhesive tape
placed approximately 1 cm below the tail tip.
The immobility time was recorded with a video tracking system.
Forced Swim Test (FST)
The forced swim test was performed according to the previously published approach [38]. Mice were placed in Plexiglas cylinders (50 cm height × 20 cm internal diameter) filled with water (23°C-25°C) to a depth of 15 cm water. The immobility time was recorded with a video tracking system over the 6 min session.
Western blot
Western blot was performed to detect the protein levels of BDNF, TrkB and p-TrkB in the frontal lobe and the hippocampal region of the mice. The frontal lobe and the hippocampal region were homogenized in a RIPA solution containing a complete protease inhibitor cocktail using an ultrasound machine. The homogenates were then centrifuged at 12,000 rpm at 4°C for 30 min and supernatants were collected and the protein concentrations were determined by a BCA detection kit (Beyotime, China). Eventually, the protein samples were combined 1:1 with 2 loading buffer and boiled with 95°C for 15 min for the following assay.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA assay was performed to detect the level of dopamine receptor and serotonin in the frontal lobe and the hippocampal region of the mice by following the manufacturer’s protocol.
Statistical analysis
GraphPad Prism 8.0.1 was used for statistical analysis and graph plotting. Student’s t-test was used for analyzation of significant differences between two sets of data. The differences in multiple sets of data were analyzed through one-way ANOVA. All data are presented as mean ± SEM. Significance levels were indicated as *p<0.05, **p<0.01, ***p<0.001.
Results
Long-term exposure of HH, HM, or CN induced depression-like behaviors in mice
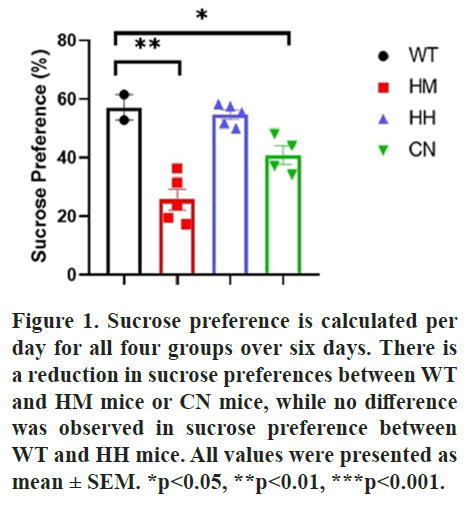
Sucrose preference, TST and FST tests were performed to evaluate the depression-like behaviors in mice. For Sucrose preference, the test was conducted per day for all groups over six days. The results showed that a significant reduction in sucrose preferences between Wild-Type (WT) and Homozygous (HM) mice (p<0.01) or CN mice (p<0.05), while no difference was observed in sucrose preference between WT and HH mice (Figure 1).
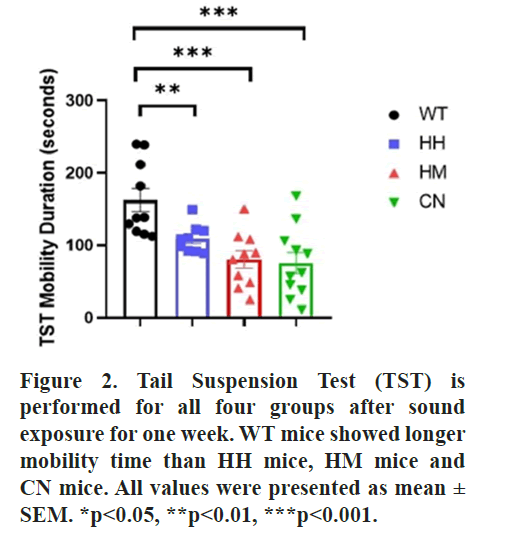
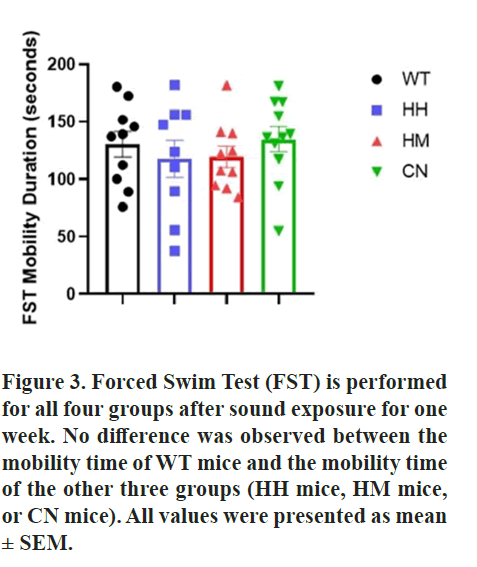
Furthermore, TST and FST tests were conducted after sound exposure for one week. Notably, results of TST also indicated that WT mice showed a significantly greater mobility time than HH mice (p<0.01), HM mice (p<0.001) and CN mice (p<0.001) while in FST, no difference was observed between the mobility time of WT mice and the mobility time of the other three groups (HH mice, HM mice, or CN mice) (Figures 2 and 3).
All results thus demonstrated that long-term exposure of HH mice, HM mice, or CN mice induced depression-like behaviors in mice. Nest Building (NB) behavioral test is also performed due to its correlation with the detrimental state of mice. No difference was observed between the quality of nest built by WT mice and the nest built by the other three groups (HH mice, HM mice, or CN mice) (Figure 4) [39].
Long-term exposure of HH, HM, or CN alleviated the fear and anxiety-like behaviors of mice
Additionally, Elevated Plus Maze (EPM) the Open Field Test (OFT) were also performed after sound exposure for one week to evaluate the fear and anxiety-like behaviors towards the new environment. In the EPM, WT mice showed significantly less time spent in open arms than HH mice (p<0.05), HM mice (p<0.01) and CN mice (p<0.05). Correspondingly, the total distance traveled in the open arm is also significantly less for WT mice than for HH mice (p<0.05), HM mice (p<0.01), CN mice (p<0.05) (Figure 5). Moreover, HM mice showed a non-significant increase in time spent in the center and distance traveled compared to the WT mice (Figure 6).
All results suggested a prevention of anxiety and fear induced by a long-term exposure of strong acoustic stimuli including HH, HM and CN.
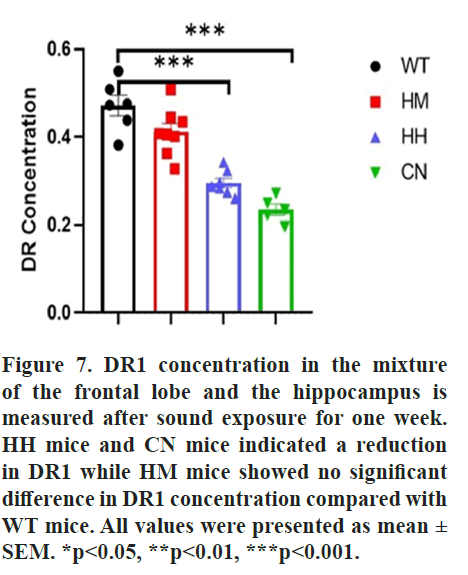
Reduction of DR1 and BDNF protein might be responsible for long-term exposure of strong acoustic stimuli-induced emotional changes
Dopamine Receptor 1 (DR1) mediates behavior related to anxiety and depression. Given that, the protein level of DR1 was detected to explore the underlying mechanism of long-term exposure of strong acoustic stimuli-induced emotional changes. Results of ELISA indicated long-term exposure of HH (p<0.001) or CN (p<0.001) reduced the protein level of DR1 in brain of mice, while an insignificant decrease in DR1 concentration between WT mice and HM mice were observed. (Figure 7).
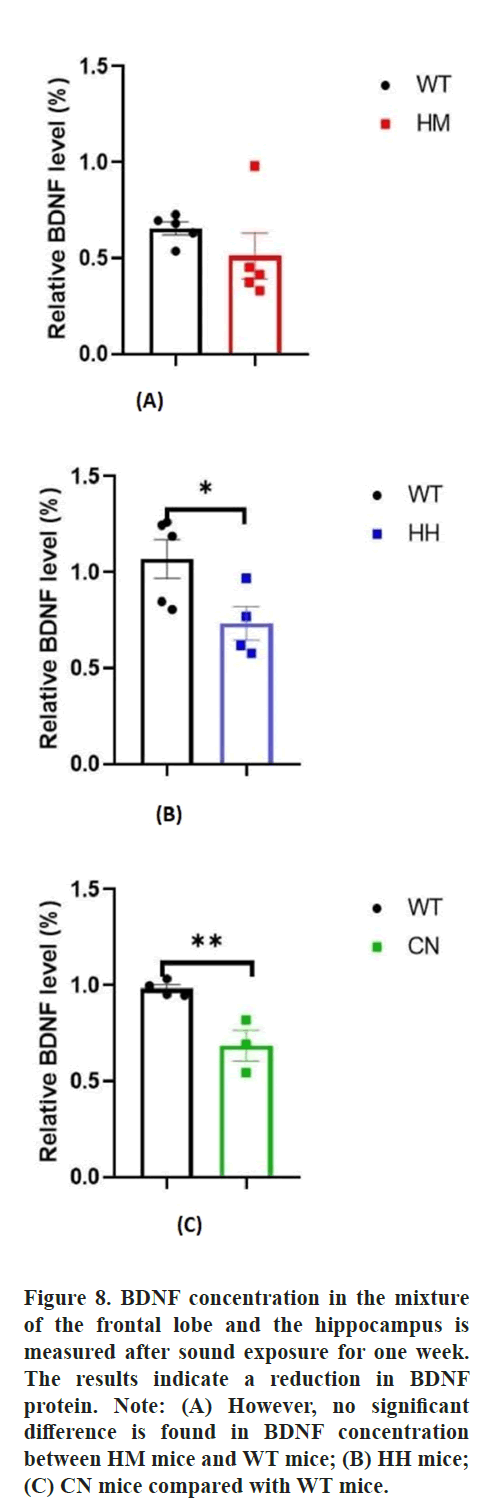
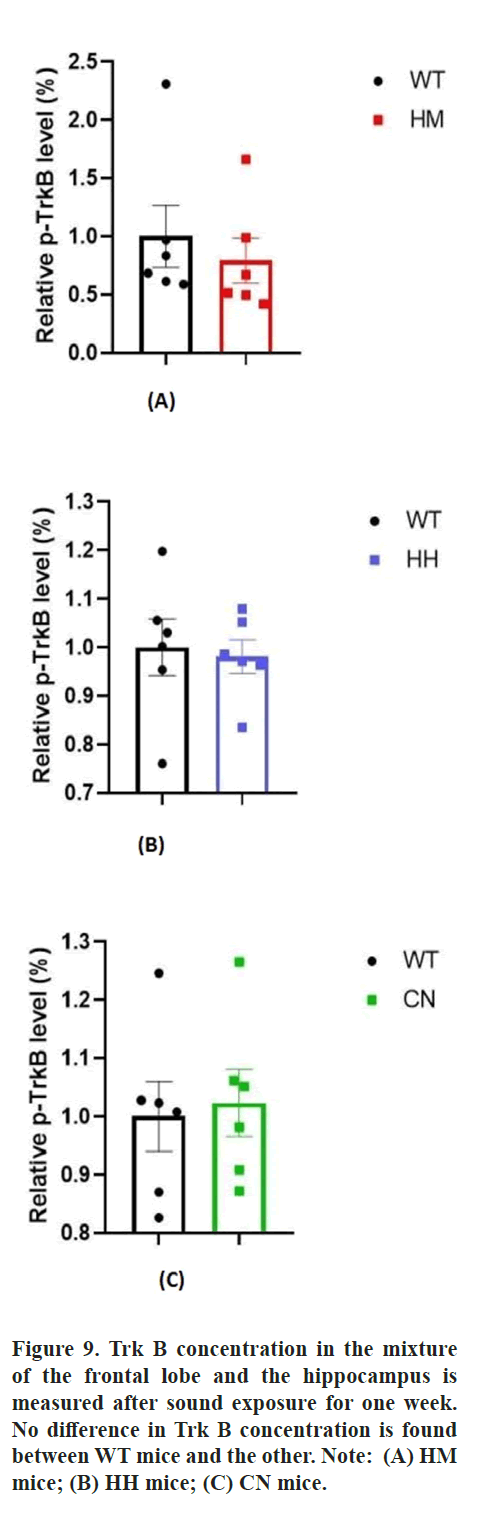
Additionally, BDNF, as a key neurotrophic factor, was also considered to be closely related to emotional changes in humans. Then, the effect of long-term exposure of strong acoustic stimuli on BDNF/Trk B pathway was also detected by western blot assay. The results showed that long-term exposure of HH (p<0.05) and CN (p<0.01) significantly reduced the protein level of BDNF protein in brain of mice and there was no difference In BDNF protein level between HM mice and WT mice (Figure 8). Unexpectedly, no difference in Tropomyosin receptor kinase B (Trk B) level between WT mice and all other three experiment groups (HM mice, HH mice, CN mice) (Figure 9).
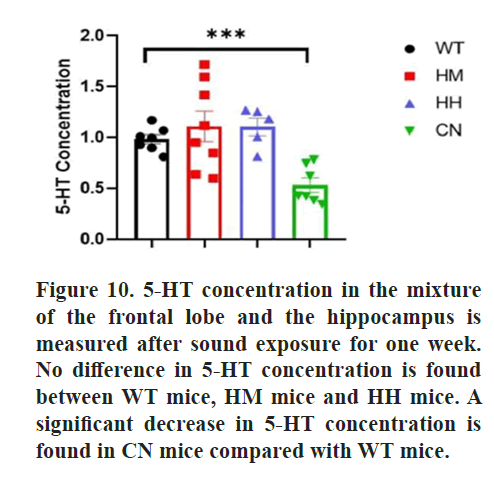
Finally, the effect of long-term exposure of strong acoustic stimuli on 5-HT level was also detected due to its involvement in emotional regulation. However, no significant difference was found in 5-HT concentration between WT mice and HM mice or HH mice, while a significant decrease in 5-HT concentration for CN mice was observed (p<0.001) (Figure 10).
Discussion
The main results of this study were that the application of long-term acoustic stimulus, including HH, HM and CN, might induce depression-like behaviors as proven by a significant decrease in sucrose preferences in HM and CN mice, a significant decrease in mobility time in the tail suspension test for HM, HH, and CN mice. Notably, long-term exposure of HH, HM and CN also alleviated the fear and anxiety-like behaviors of mice as indicated by an increase in time spent and distance traveled in the open arm in the elevated plus maze. Furthermore, our results suggested a significant reduction in DR1 and BDNF protein levels in the hippocampus and frontal lobe might be responsible for long-term exposure of strong acoustic stimuli-induced emotional changes.
The expression level of D1R is related to stress stimuli. Chronic stress exposure decreases dopamine levels in the mPFC (medial prefrontal cortex). Repeated social defeat stress reduces the mRNA level of D1 receptors in mPFC, which also plays a significant role in suppressing stress susceptibility [40,41].
BDNF protein is also influenced by stress stimuli. Early life stress may exert differential alterations to the expression of BDNF protein and CREB transcripts in the hippocampus, contributing to individual differences in hippocampal vulnerability to stress which influences mood. Chronic social defeat stress induces lasting down regulation of BDNF protein which is reversed by the anti-depressant imipramine [42]. Chronic stress reduces the expression of BDNF protein in the dentate gyrus to induce neurogenesis in the hippocampus, preventing depression, but does not affect the expression of trkB, which may explain the reduction in BDNF protein accompanied by a constant concentration of trkB for HH and CN mice [43-45].
D1-like receptors, which include D1R and D5R, are associated with the regulation of depressive symptoms. It has been proven that D1 but not D2 receptor activation increases protein synthesis by eEF2 (Eukaryotic elongation factor 2) dephosphorylation through inhibiting of eEF2K (eEF2 kinase). Notably, the anti-depressant effects of ketamine are also attributed to the inhibition of spontaneous glutamate release-driven N-Methyl-D-Aspartate (NMDA) receptor activity, following by a decrease in eEF2K activity, thus increasing protein synthesis [46]. This implies that DR1’s involvement in the regulation of depressive symptoms might be similar to that of ketamine. Moreover, a strong connection is established between ketamine and DR1 in the sense that acute ketamine administration is associated with significantly increased dopamine levels in the cortex [47]. Therefore, DR1 has been proposed as a possible target for treatments of depression, which yields promising results. Similarly, D1-receptor stimulation by D1 receptor agonists is shown to relieve pain-related depression [48]. Injections of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which reduces dopaminergic neurons, impairs the DR1-mediated neurogenesis in v-DG (ventral dentate gyrus) in the hippocampus, induced depression-like symptoms [49]. Moreover, the addiction to abuse quetiapine (an effective atypical antipsychotic in the management of mental disorders including depression and anxiety) is blocked by pretreatment of D1 receptor antagonist, suggesting the modulatory role of D1 receptor in the anti-depressant pathway of quetiapine [50,51].
BDNF protein level is also related to the mediation of the depression pathway from previous research. Studies prove that the up-regulation of BDNF protein level in the hippocampus produced anti-depressant effects. Serum BDNF protein is lower in patients with major depressed patients and anti-depressant treatments restore BDNF protein level [52-55]. However, the mechanisms of the anti-depressive role of BDNF protein are still unclear [56].
Nevertheless, the role that BDNF protein plays in the depression pathway is ambiguous; it may be required for activity-dependent plasticity linked to depression. The simultaneous decreases in DR1 and BDNF protein for HH and CN mice suggested a possible relationship between BDNF protein and DR1. SKF 83959, a D5R and D1-D2 receptor heteromer agonist, induces a 70% increase in the expression of BDNF protein in the PFC (prefrontal cortex) and elevates Akt signaling, acting like an anti-depressant [57,58]. Nevertheless, controversial evidence explains how the activation of dopamine D1-D2 receptor heteromer by dopamine or agonist SKF 83959 leads to intracellular calcium mobilization, resulting in CaMKIIα activation which leads to enhanced BDNF protein production in the nucleus accumbens, while BDNF protein exerts anti-depressant-like effects in the hippocampus and pro-depressant effects in the nucleus accumbens [59]. The interrelation between BDNF protein and DR1 can exert opposite influences on mood disorders depending on specific regions of the brain, but the connection between the two is evident [52].
The reduction of DR1 in the mixture of the hippocampus and frontal lobe can be responsible for the anxiolytic effects of acoustic stimuli. A dopaminergic pathway, such as the projection of the ventral tegmentum area to the interpeduncular nucleus, is found to mediate anxiety-like behaviors. Specifically, the excitation of the release of dopamine in Ventral Tegmental Area (VTA) to Interpeduncular Nucleus (IPN) increased, while the inhibition of this pathway reduced, the anxiety behaviors. Nicotine, a dopamine agonist that stimulates dopamine neurons of the ventral tegmentum area, induces anxiety [60,61].
The decrease in anxiety suggested by the behavioral tests may also be related to the corresponding decrease in BDNF protein level. The BDNF protein -TrkB pathway is essential for the consolidation of fear conditioning in the amygdala, as BDNF protein deletions greatly impair the consolidation of fear conditioning. A Single-Nucleotide Polymorphism (SNP) in the BDNF gene (BDNF Val66Met) that produces Met/Met rats demonstrates a decrease in BDNF protein secretion and a deficit in forming fear memory [62,63]. Additionally, under a stressful setting, Met/Met mice exhibited increased anxiety-related behaviors [64]. These findings suggest that anxiety is influenced by the status of the BDNF Val/Met allele [65].
Furthermore, a relationship is found between BDNF protein and serotonin (5-HT). The decrease in basal BDNF protein levels in the hippocampus is not reversed with the administration of fluoxetine, a Selective Serotonin Reuptake Inhibitor (SSRI) that normally produce anxiolytic and anti-depressant effects, suggesting that the function of SSRIs may depend on BDNF protein [66]. The reduction in BDNF due to BDNF gene polymorphism results in increased anxiety behaviors and decreased 5-HT fiber density. This may explain the anxiolytic effect of construction noise, exemplified by a decrease in time spent and total distance traveled in the open arm during the EPM and a significant decrease in BDNF protein level and 5-HT level [67]. However, the anxiolytic effects of music including heavy metal and hip-hop show more correlation with the decrease in BDNF protein concentration and are not related to 5-HT level.
This study has several limitations. The first limitation was the time frame. One week of acoustic stimuli might not be enough to induce any significant mental disorder or verify the long-term effects of HH, HM, or CN on adolescents. Another limitation was the exclusiveness of the male sex. Female mice may yield completely different results. For adult ovariectomized female rats, dopamine agonists produced depressive-like symptoms while dopamine antagonists exerted anti-depressive-like effects, implying the complexity of dopamine receptor’s role in inducing or reducing depression depending on different gender [68]. Additionally, female patients were more depressed and expressed less BDNF. The disorder of D1-D2 receptor heteromer in females may significantly promote females’ susceptibility to depression and anxiety disorders [54]. Lastly, this study slightly explored the possibility of inducing anxiety or depression through HH, HM, or CN and other mental disorders and molecular changes untested might have taken place [69].
Additionally, dopamine acts at two different receptor families: D1-like receptors (D1 and D5) and D2-like receptors (D2, D3 and D4). This study focused solely on D1-like receptors, while D2-like receptors are also involved in depression, especially for D2 and D3. Several DR2 and DR3 agonists, including 7-Hydroxy-2-(di-n-propylamino)tetralin (7-OH-DPAT), BP 897 and pramipexole, produced anxiolytic- and anti-depressant-like effects in the animal model. D3R deficiency also results in chronic depression and anxiety [70,71]. Moreover, the down regulation of D3R in the nucleus accumbens shifted microglia to the pro-inflammatory stage and contributes to the development of depressive-like behaviors through Akt signaling pathway, marking a shared pathway that is regulated by both DR1 and DR3 [72,73]. Therefore, further experiments should be performed to measure different dopamine receptors, D1-like or D2-like, in different brain regions, hippocampus or nucleus accumbens, to determine the role of acoustic stimuli in inducing depression or anxiety [74].
Conclusion
In conclusion, acoustic stimuli (heavy metal music, hip-hop music and construction noise) applied chronically over the course of a week induces depression while alleviates anxiety in 8 weeks old mice models. The sound stimuli also reduce the amount of dopamine receptors 1 and BDNF protein in the mixture of the frontal lobe and hippocampus. Nevertheless, additional research needs to confirm this finding due to its limited time period and exclusiveness on males. Further research waits to be conducted to ascertain the relationship between the decrease in protein on a molecular level and the observed behaviors related to depression and anxiety.
Authors Contributions
Jian Lu and Jingyao Ren designed the study, performed the animal and cell experiments, and analyzed the interpreted data. Jingyao Ren wrote the manuscript and Jian Lu revised it. Both authors approved the manuscript.
Conflict of Interest Statement
The authors declare that they have no conflict of interest. The care and use of laboratory animals were conducted in accordance with all institutional and national guidelines.
Data Availability Statement
Upon a reasonable request, the corresponding author will provide the data sets used and/or analyzed in the current study.
References
- Lin ST, Yang P, Lai CY, Su YY, Yeh YC, et al. Mental health implications of music: Insight from neuro-scientific and clinical studies. Harv Rev Psychiatry. 2011;19(1):34-46.
[Crossref] [Google Scholar] [PubMed]
- Fu Q, Qiu R, Chen L, Chen Y, Qi W, et al. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl Psychiatry. 2023;13(1):317.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Wei Y, Yang W, Jiang L, Li X, et al. The effectiveness of music therapy for terminally ill patients: A meta-analysis and systematic review. J Pain Symptom Manage. 2019;57(2):319-329.
[Crossref] [Google Scholar] [PubMed]
- Erkkila J, Punkanen M, Fachner J, Ala-Ruona E, Pontio I, et al. Individual music therapy for depression: Randomised controlled trial. Br J Psychiatry. 2011;199(2):132-139.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Wang R, Tang X, Li Q, Xu G, et al. The effect of music, massage, yoga and exercise on antenatal depression: A meta-analysis. J Affect Disord. 2021;292:592-602.
[Crossref] [Google Scholar] [PubMed]
- Mossler K, Chen X, Heldal TO, Gold C. Music therapy for people with schizophrenia and schizophrenia‐like disorders. Cochrane Database Syst Rev. 2017;5(5):CD004025.
[Crossref] [Google Scholar] [PubMed]
- Bidabadi SS, Mehryar A. Music therapy as an adjunct to standard treatment for obsessive compulsive disorder and co-morbid anxiety and depression: A randomized clinical trial. J Affect Disord. 2015;184:13-17.
[Crossref] [Google Scholar] [PubMed]
- Elliott D, Polman R, McGregor R. Relaxing music for anxiety control. J Music Ther. 2011;48(3):264-288.
[Crossref] [Google Scholar] [PubMed]
- Recours R, Aussaguel F, Trujillo N. Metal music and mental health in France. Cult Med Psychiatry. 2009;33(3):473-488.
[Crossref] [Google Scholar] [PubMed]
- Baker F, Bor W. Can music preference indicate mental health status in young people? Australas Psychiatry. 2008;16(4):284-288.
[Crossref] [Google Scholar] [PubMed]
- Miranda D, Claes M. Rap music genres and deviant behaviors in French-Canadian adolescents. J Youth Adolesc. 2004;33:113-122.
[Crossref] [Google Scholar] [PubMed]
- Arnett J. Adolescents and heavy metal music: From the mouths of metalheads. J Youth Adolesc. 1991;23(1):76-98.
[Crossref] [Google Scholar] [PubMed]
- Hines M, McFerran KS. Metal made me who I am: Seven adult men reflect on their engagement with metal music during adolescence. Int J of Community Musi. 2014;7(2):205-222.
[Crossref] [Google Scholar]
- Martin G, Clarke M, Pearce C. Adolescent suicide: Music preference as an indicator of vulnerability. J Am Acad Child Adolesc Psychiatry. 1993;32(3):530-535.
[Crossref] [Google Scholar] [PubMed]
- Baker C, Brown B. Suicide, self-harm and survival strategies in contemporary heavy metal music: A cultural and literary analysis. J Med Humanit. 2016;37(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- Gong X, Fenech B, Blackmore C, Chen Y, Rodgers G, et al. Association between noise annoyance and mental health outcomes: A systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(5):2696.
[Crossref] [Google Scholar] [PubMed]
- Peng X, Mao Y, Tai Y, Luo B, Dai Q, et al. Characterization of anxiety-like behaviors and neural circuitry following chronic moderate noise exposure in mice. Environ Health Perspect. 2023;131(10):107004.
[Crossref] [Google Scholar] [PubMed]
- Hegewald J, Schubert M, Freiberg A, Romero Starke K, Augustin F, et al. Traffic noise and mental health: A systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(17):6175.
[Crossref] [Google Scholar] [PubMed]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863.
[Crossref] [Google Scholar] [PubMed]
- Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979;163(2):195-205.
[Crossref] [Google Scholar] [PubMed]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, et al. Entering adolescence: Resistance to peer influence, risky behavior and neural changes in emotion reactivity. Neuron. 2011;69(5):1029-1036.
[Crossref] [Google Scholar] [PubMed]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565-1582.
[Crossref] [Google Scholar] [PubMed]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947-957.
[Crossref] [Google Scholar] [PubMed]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189-1201.
[Crossref] [Google Scholar] [PubMed]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34(5):631-648.
[Crossref] [Google Scholar] [PubMed]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience. 2013;249:242-257.
[Crossref] [Google Scholar] [PubMed]
- Anisman H, Merali Z, Stead JD. Experiential and genetic contributions to depressive-and anxiety-like disorders: Clinical and experimental studies. Neurosci Biobehav Rev. 2008;32(6):1185-1206.
[Crossref] [Google Scholar] [PubMed]
- Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, et al. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193(2):311-314.
[Crossref] [Google Scholar] [PubMed]
- Gameiro GH, Gameiro PH, da Silva Andrade A, Pereira LF, Arthuri MT, et al. Nociception-and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav. 2006;87(4):643-649.
[Crossref] [Google Scholar] [PubMed]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667-673.
[Crossref] [Google Scholar] [PubMed]
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13(7):1686-1698.
[Crossref] [Google Scholar] [PubMed]
- Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1(3):1117-1119.
[Crossref] [Google Scholar] [PubMed]
- Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Pre-clinical Models. 2019;1916:99-103.
[Crossref] [Google Scholar] [PubMed]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525-529.
[Crossref] [Google Scholar] [PubMed]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, et al. The tail suspension test. J Vis Exp. 2012;(59):e3769.
[Crossref] [Google Scholar] [PubMed]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: A new model sensitive to anti-depressant treatments. Eur J Pharmacol. 1978;47(4):379-391.
[Crossref] [Google Scholar] [PubMed]
- Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139-146.
[Crossref] [Google Scholar] [PubMed]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, et al. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting anti-depressant effects. Nat Commun. 2019;10(1):223.
[Crossref] [Google Scholar] [PubMed]
- Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry. 2018;23(8):1717-1730.
[Crossref] [Google Scholar] [PubMed]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, et al. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32(7):1504-1519.
[Crossref] [Google Scholar] [PubMed]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and anti-depressant action. Nat Neurosci. 2006;9(4):519-525.
[Crossref] [Google Scholar] [PubMed]
- Warner‐Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and anti-depressant treatment. Hippocampus. 2006;16(3):239-249.
[Crossref] [Google Scholar] [PubMed]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15(3):1768-1777.
[Crossref] [Google Scholar] [PubMed]
- David O, Barrera I, Gould N, Gal-Ben-Ari S, Rosenblum K. D1 dopamine receptor activation induces neuronal eEF2 pathway-dependent protein synthesis. Front Mol Neurosci. 2020;13:67.
[Crossref] [Google Scholar] [PubMed]
- Adaikkan C, Taha E, Barrera I, David O, Rosenblum K. Calcium/calmodulin-dependent protein kinase II and eukaryotic elongation factor 2 kinase pathways mediate the anti-depressant action of ketamine. Biol Psychiatry. 2018;84(1):65-75.
[Crossref] [Google Scholar] [PubMed]
- Kokkinou M, Ashok AH, Howes OD. The effects of ketamine on dopaminergic function: Meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry. 2018;23(1):59-69.
[Crossref] [Google Scholar] [PubMed]
- Lazenka MF, Freitas KC, Henck S, Negus SS. Relief of pain-depressed behavior in rats by activation of D1-like dopamine receptors. J Pharmacol Exp Ther. 2017;362(1):14-23.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Hong J, Di T, Chen L. MPTP impairs dopamine D1 receptor-mediated survival of newborn neurons in ventral hippocampus to cause depressive-like behaviors in adult mice. Front Mol Neurosci. 2016;9:101.
[Crossref] [Google Scholar] [PubMed]
- Althobaiti YS. Quetiapine-induced place preference in mice: Possible dopaminergic pathway. Pharmaceuticals. 2021;14(2):156.
[Crossref] [Google Scholar] [PubMed]
- Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of anti-depressant therapies. Pharmacol Ther. 2008;117(1):30-51.
[Crossref] [Google Scholar] [PubMed]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces anti-depressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251-3261.
[Crossref] [Google Scholar] [PubMed]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143-148.
[Crossref] [Google Scholar] [PubMed]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, et al. Alterations of serum levels of Brain-Derived Neurotrophic Factor (BDNF) in depressed patients with or without anti-depressants. Biol Psychiatry. 2003;54(1):70-75.
[Crossref] [Google Scholar] [PubMed]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by anti-depressant treatments in the adult rat brain. Neuropharmacology. 2003;45(4):553-563.
[Crossref] [Google Scholar] [PubMed]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18-21.
[Crossref] [Google Scholar] [PubMed]
- Perreault ML, Jones-Tabah J, O’Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2013;16(2):477-483.
[Crossref] [Google Scholar] [PubMed]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, et al. Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci. 2009;106(50):21377-21382.
[Crossref] [Google Scholar] [PubMed]
- DeGroot SR, Zhao-Shea R, Chung L, Klenowski PM, Sun F, et al. Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biol Psychiatry. 2020;88(11):855-866.
[Crossref] [Google Scholar] [PubMed]
- Nguyen C, Mondoloni S, Le Borgne T, Centeno I, Come M, et al. Nicotine inhibits the VTA-to-amygdala dopamine pathway to promote anxiety. Neuron. 2021;109(16):2604-2615.
[Crossref] [Google Scholar] [PubMed]
- Heldt SA, Zimmermann K, Parker K, Gaval M, Ressler KJ. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34(7):2444-2450.
[Crossref] [Google Scholar] [PubMed]
- Dincheva I, Pattwell SS, Tessarollo L, Bath KG, Lee FS. BDNF modulates contextual fear learning during adolescence. Dev Neurosci. 2014;36(3-4):269-276.
[Crossref] [Google Scholar] [PubMed]
- Jaehne EJ, Kent JN, Antolasic EJ, Wright BJ, Spiers JG, et al. Behavioral phenotyping of a rat model of the BDNF Val66Met polymorphism reveals selective impairment of fear memory. Transl Psychiatry. 2022;12(1):93.
[Crossref] [Google Scholar] [PubMed]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140-143.
[Crossref] [Google Scholar] [PubMed]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, et al. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37(5):1297-1304.
[Crossref] [Google Scholar] [PubMed]
- Duman RS. BDNF, 5-HT and anxiety: Identification of a critical periadolescent developmental period. Am J Psychiatry. 2017;174(12):1137-1139.
[Crossref] [Google Scholar] [PubMed]
- Fedotova J, Ordyan N. Involvement of D1 receptors in depression-like behavior of ovariectomized rats. Acta Physiol Hung. 2011;98(2):165-176.
[Crossref] [Google Scholar] [PubMed]
- Hasbi A, Nguyen T, Rahal H, Manduca JD, Miksys S, et al. Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression-and anxiety-like behaviors. Biol Sex Differ. 2020;11(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Hori H, Kunugi H. The efficacy of pramipexole, a dopamine receptor agonist, as an adjunctive treatment in treatment‐resistant depression: An open‐label trial. Sci World J. 2012;2012(1):372474.
[Crossref] [Google Scholar] [PubMed]
- Rogoz Z, Skuza G, Klodzinska A. Anxiolytic-and anti-depressant-like effects of 7-OH-DPAT, preferential dopamine D3 receptor agonist, in rats. Pol J Pharmacol. 2004;56(5):519-526.
[Google Scholar] [PubMed]
- Moraga-Amaro R, Gonzalez H, Pacheco R, Stehberg J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behav Brain Res. 2014;274:186-193.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Lai S, Wang R, Zhou T, Dong N, et al. Dopamine D3 receptor in the nucleus accumbens alleviates neuro-inflammation in a mouse model of depressive-like behavior. Brain Behav Immun. 2022;101:165-179.
[Crossref] [Google Scholar] [PubMed]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, et al. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behavioural brain research. 2005;162(1):127-134.
[Crossref] [Google Scholar] [PubMed]
Citation: Heavy metal Music, Hip-hop Music and Construction Noise Induces Depressive Symptoms in mice ASEAN Journal of
Psychiatry, Vol. 25 (8) October, 2024; 1-14.