FACTORS CONTRIBUTING TO DIFFERENCES IN THE ANTICONTRACTILE FUNCTION OF THORACIC AND ABDOMINAL PERIVASCULAR ADIPOSE TISSUE
Department of Physical Education, Guangzhou College of Commerce, Guangzhou, China
*Corresponding Author:
Erling Guo,
Department of Physical Education, Guangzhou College of Commerce, Guangzhou,
China,
Email: gel2022@163.com
Received: 13-Nov-2024, Manuscript No. AJOPY-24-153797;
Editor assigned: 15-Nov-2024, Pre QC No. AJOPY-24-153797 (PQ);
Reviewed: 29-Nov-2024, QC No. AJOPY-24-153797;
Revised: 06-Dec-2024, Manuscript No. AJOPY-24-153797 (R);
Published:
13-Dec-2024, DOI: 10.54615/2231-7805.47384
About the Study
Perivascular Adipose Tissue (PVAT) is a unique ectopic fat depot that attaches to the majority of blood vessels. Initially, it is regarded solely as a structural supportive tissue that provides physical protection for blood vessels [1]. However, PVAT noted for maintaining vascular homeostasis because of its proximity to and direct contact with the adventitia which is considered as its foremost function [2,3]. This specialized tissue secretes adipokines with vasodilatory functions and regulates vascular function via paracrine and endocrine mechanisms [4-6]. Interestingly, PVAT exhibits phenotypic differences depending on its anatomical location. Specifically, thoracic PVAT consists of Brown Adipose Tissue (BAT), whereas abdominal PVAT comprises a combination of both BAT and White Adipose Tissue (WAT) [7,8]. PVATs in different regions exhibit significant variations not only in phenotype, but also in the number and size of adipocytes, the degree of immune cell infiltration, the response to various agonists, the ability to release specific adipokines, Norepinephrine (NE) concentrations and innervation. The considerable heterogeneity between thoracic and abdominal PVAT may lead to regional variations in anticontractile function.
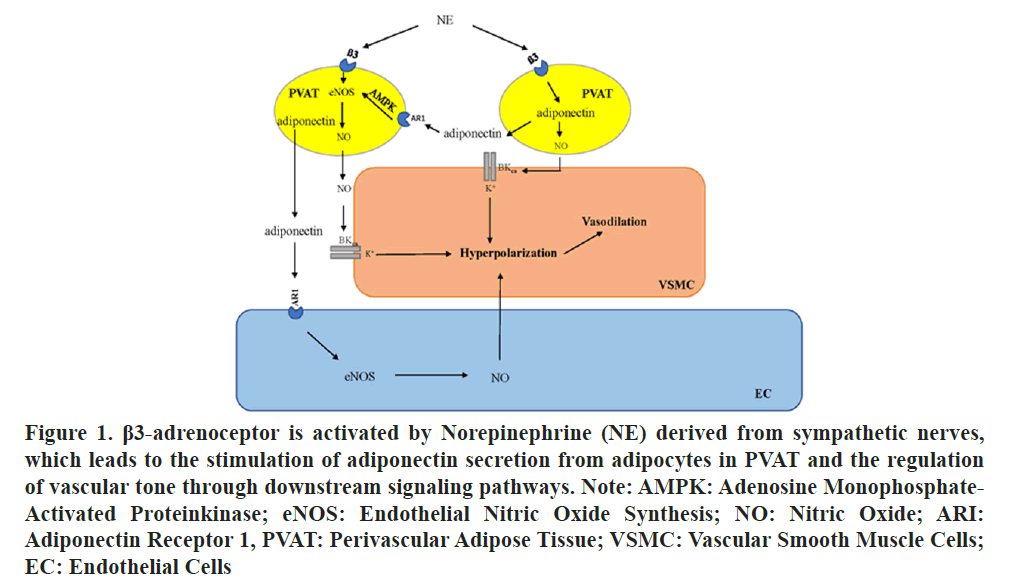
Previous studies have demonstrated that the number and size of abdominal adipocytes are significantly greater than those of thoracic adipocytes and the levels of inflammatory genes and immune cell infiltration markers in abdominal PVAT are greater than those in thoracic PVAT. The pro inflammatory and proatherosclerotic characteristics of abdominal PVAT are more pronounced than those of thoracic PVAT, potentially accounting for the heightened susceptibility of the abdominal aortic region to the disease [9]. The differences in histology and the immune cell response of thoracic and abdominal PVAT may be associated with regional differences in PVAT-mediated vascular function. in the rat aorta it was observed that PVAT diminishes the contractile response to NE, this is the major role of PVAT in vascular function [10]. However, the heterogeneity of PVAT across various aortic regions results in regional differences in the modulation of vascular function. The anticontractile function was found to be impaired in the abdominal PVAT group compared with the thoracic aorta group. Victorio et al., conducted an analysis of the mechanisms responsible for regional differences in the anticontractile function of PVAT by comparing Nitric Oxide (NO) synthesis and availability. Reactive Oxygen Species (ROS) production and lipid peroxidation were found to be comparable between the abdominal and thoracic aorta as well as between abdominal and thoracic PVAT, whereas the expression of endothelial Nitric Oxide Synthase (eNOS) and NO utilization in the abdominal PVAT are notably lower compared to those in thoracic PVAT, with no significant changes observed in the vasculature [11]. Hence, regional disparities in NO production derived from eNOS in PVAT adipocytes might contribute to the variations in their anticontractile function. However, a previous study demonstrated that the inhibition of NOS does not block the anticontractile function of aortic PVAT. Therefore, it can potentially be deduced that the relaxation factor derived from PVAT is not NO [12]. When PVAT is stimulated, it releases a transferable anticontractile factor known as adiponectin, it is evident from the experiments with organ bath solution transfer. NO enhances the opening of large-conductance calcium- and voltage-activated K+ channels BKCa in Vascular Smooth Muscle Cells (VSMCs) derived by adipocyte resulting in membrane hyperpolarization and subsequent vasodilation. The release of the vasodilator adiponectin from PVAT is initiated by the activation of the β3-adrenergic receptor. This mechanism is mediated by the sympathetic neurotransmitter NE, a sympathetic neurotransmitter as shown in Figure 1 [5,13-16].
Interestingly, similar regional disparities in the concentration of NE within adipose tissue have been observed. Ahmad et al., conducted an analysis of the content of NE in thoracic PVAT, superior mesenteric PVAT, mesenteric PVAT and retroperitoneal fat and reported that thoracic PVAT, which contains more brown adipocytes, had a greater content of NE; that mesenteric PVAT, which contains more white adipocytes, had a lower content of NE. The NE content in thoracic PVAT was approximately 7-fold higher than that in mesenteric PVAT. Furthermore, the NE content in superior mesenteric PVAT was intermediate between the levels observed in thoracic and mesenteric PVA. The variations in NE content between different tissues may be associated with differences in the adipose tissue phenotype [17]. Thus, the abdominal PVAT, which comprises a mixture of brown and white tissue, may have lower levels of NE than the thoracic PVAT dose [9]. Additionally, NE is a sympathetic neurotransmitter and because it stimulates the anticontractile function of PVAT, there is substantial evidence that PVAT is innervated by sympathetic nerves [10,13,18-20]. Similarly, the nerve density in adipose tissue varies regionally. Contreras et al., elucidated the major role of nerve density in facilitating the development of the brown adipocyte phenotype. Compared with WAT, BAT contains a greater number of sympathetic fibers [21-23]. Analysis of the innervation density of aortic PVAT, mesenteric PVAT and WAT revealed that aortic PVAT, which contains brown adipocytes, is densely innervated by sympathetic nerves, whereas mPVAT, which consists of white adipocytes and WAT are less densely innervated.
Therefore, the innervation density of brown-like thoracic PVAT depots are found to be more than that of abdominal PVAT depots, comprising of a mixture of brown and white tissues. It could be a complex phenomenon associated with the elevated NE content in thoracic PVAT. NE concentrations and Sympathetic innervation were found to reveal regional variations within PVAT and the release of anticontractile factors from PVAT is driven by processes downstream of the NE-mediated stimulation of adipocytes [16]. Variations in NE content between thoracic and abdominal PVAT may influence regional disparities in anticontractile function by affecting the activity of the β3-adiponectin-eNOS pathway.
References
- Antoniades C, Tousoulis D, Vavlukis M, Fleming I, Duncker DJ, et al. Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers. Eur Heart J. 2023;44(38):3827-3844.
[Crossref][Google Scholar][PubMed]
- Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, et al. Regulation of vascular function and insulin sensitivity by adipose tissue: Focus on perivascular adipose tissue. Microcirculation. 2007;14(4-5):389-402.
[Crossref][Google Scholar][PubMed]
- Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951-968.
[Crossref][Google Scholar][PubMed]
- Szasz T, Webb RC. Perivascular adipose tissue: More than just structural support. Clin Sci (Lond). 2012;122(1):1-12.
[Crossref][Google Scholar][PubMed]
- Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, et al. Stimulated release of a hyperpolarizing factor ADHF from mesenteric artery perivascular adipose tissue: Involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol. 2013;169(7):1500-1509.
[Crossref][Google Scholar][PubMed]
- Araujo HN, da Silva CP, Sponton AC, Clerici SP, Davel AP, et al. Perivascular adipose tissue and vascular responses in healthy trained rats. Life Sci. 2015;125:79-87.
[Crossref][Google Scholar][PubMed]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151(3):323-331.
[Crossref][Google Scholar][PubMed]
- Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, et al. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34(8):1621-1630.
[Crossref][Google Scholar][PubMed]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29(10):1458-1464.
[Crossref][Google Scholar][PubMed]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13(2):277-296.
[Crossref] [Google Scholar] [PubMed]
- Victorio JA, Fontes MT, Rossoni LV, Davel AP. Different anti-contractile function and nitric oxide production of thoracic and abdominal perivascular adipose tissues. Front Physiol. 2016;7:295.
[Crossref][Google Scholar][PubMed]
- Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, et al. Periadventitial fat releases a vascular relaxing factor. Faseb J. 2002;16(9):1057-1063.
[Crossref] [Google Scholar] [PubMed]
- Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, et al. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2018;38(4):880-891.
[Crossref][Google Scholar][PubMed]
- Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM. cGMP-dependent Protein Kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014;101(1):130-137.
[Crossref][Google Scholar][PubMed]
- Withers SB, Bussey CE, Saxton SN, Melrose HM, Watkins AE, et al. Mechanisms of adiponectin-associated perivascular function in vascular disease. Arterioscler Thromb Vasc Biol. 2014;34(8):1637-1642.
[Crossref][Google Scholar][PubMed]
- Hanscom M, Morales-Soto W, Watts SW, Jackson WF, Gulbransen BD. Innervation of adipocytes is limited in mouse perivascular adipose tissue. Am J Physiol Heart Circ Physiol. 2024;327(1):H155-H181.
[Crossref][Google Scholar][PubMed]
- Ahmad MF, Ferland D, Ayala-Lopez N, Contreras GA, Darios E, et al. Perivascular adipocytes store norepinephrine by vesicular transport. Arterioscler Thromb Vasc Biol. 2019;39(2):188-199.
[Crossref][Google Scholar][PubMed]
- Agabiti-Rosei C, de Ciuceis C, Rossini C, Porteri E, Rodella LF, et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J Hypertens. 2014;32(6):1264-1274.
[Crossref][Google Scholar][PubMed]
- Willows JW, Blaszkiewicz M, Lamore A, Borer S, Dubois AL, et al. Visualization and analysis of whole depot adipose tissue neural innervation. iScience. 2021;24(10):103127.
[Crossref][Google Scholar][PubMed]
- Perdikari A, Cacciottolo T, Henning E, Mendes de Oliveira E, Keogh JM, et al. Visualization of sympathetic neural innervation in human white adipose tissue. Open Biol. 2022;12(3):210345.
[Crossref][Google Scholar][PubMed]
- Contreras GA, Lee YH, Mottillo EP, Granneman JG. Inducible brown adipocytes in subcutaneous inguinal white fat: The role of continuous sympathetic stimulation. Am J Physiol Endocrinol Metab. 2014;307(9):E793-E799.
[Crossref][Google Scholar][PubMed]
- Sheng LJ, Ruan CC, Ma Y, Chen DR, Kong LR, et al. Beta3 adrenergic receptor is involved in vascular injury in deoxycorticosterone acetate-salt hypertensive mice. FEBS Lett. 2016;590(6):769-778.
[Crossref][Google Scholar][PubMed]
- Diculescu I, Stoica M. Fluorescence histochemical investigation on the adrenergic innervation of the white adipose tissue in the rat. J Neurovisc Relat. 1970;32(1):25-36.
[Crossref][Google Scholar][PubMed]